Human FAS / CD95 / APO-1 / TNFRSF6 Protein (Fc Tag)
ALPS1A,APO-1,APT1,CD95,FAS1,FASTM,TNFRSF6
- 100ug (NPP3722) Please inquiry
| Catalog Number | P10217-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | ALPS1A,APO-1,APT1,CD95,FAS1,FASTM,TNFRSF6 |
| Molecular Weight | The recombinant human Fas/Fc chimera is a disulfide-linked homodimeric protein generated after removal of the signal peptide. The reduced monomer consists of 386 amino acids and has a predicted molecular mass of 43.4 kDa. In SDS-PAGE under reducing conditions, the monomer migrates as an approximately 55-60 kDa protein due to glycosylation. |
| predicted N | Gln 26 |
| SDS-PAGE | 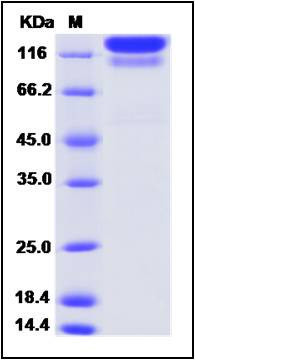 |
| Purity | (75.9+20.9) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain (Met 1-Glu 173) of human Fas antigen (NP_000034.1) was fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | Measured by its ability to inhibit Fas Ligand induced apoptosis of Jurkat human acute T cell leukemia cells. The ED50 for this effect is typically 0.02-0.1 μg/mL in the presence of 20 ng/mL recombinant human Fas ligand. |
| Research Area | Cancer |Signal transduction |Other Related Intracellular Topics |Regulation of Apoptosis by TNF Superfamily Members |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | CD95 (APO-1/Fas) is an important inducer of the extrinsic apoptosis signaling pathway and therapy induced apoptosis of many tumor cells has been linked to the activity of CD95. is a prototype death receptor characterized by the presence of an 80 amino acid death domain in its cytoplasmic tail. This domain is essential for the recruitment of a number of signaling components upon activation by either agonistic anti-CD95 antibodies or cognate CD95 ligand that initiate apoptosis. The complex of proteins that forms upon triggering of CD95 is called the death-inducting signaling complex (DISC). The DISC consists of an adaptor protein and initiator caspases and is essential for induction of apoptosis. CD95 is also crucial for the negative selection of B cells within the germinal center (GC). Impairment of CD95-mediated apoptosis results in defective affinity maturation and the persistence of autoreactive B-cell clones. Changes in the expression of CD95 and/or its ligand CD95L are frequently found in human cancer. The downregulation or mutation of CD95 has been proposed as a mechanism by which cancer cells avoid destruction by the immune system through reduced apoptosis sensitivity. Thus, CD95 has therefore been viewed as a tumor suppressor. CD95 has been reported to be involved in the activation of NF-kappaB, MAPK3/ERK1, MAPK8/JNK, and the alternate pathways for CTL-mediated cytotoxicity. Accordingly, this protein is implicated in the pathogenesis of various malignancies and diseases of the immune system. The CD95/CD95L system was implicated in the etiology of inflammatory bowel disease (IBD) based, primarily, on the finding that CD95 is highly expressed in the intestinal epithelial cells and that epithelial apoptosis is increased in IBD. |
| Reference |
