Human FDPS Protein (His Tag)
FPPS,FPS
- 100ug (NPP3857) Please inquiry
| Catalog Number | P13229-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | FPPS,FPS |
| Molecular Weight | The recombinant human FDPS consisting of 368 amino acids and has a calculated molecular mass of 42.4 kDa. It migrates as an approximately 38 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 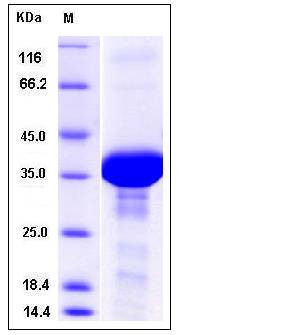 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human FDPS isoform b (NP_001129294.1) (Met 1-Lys 353) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Metabolism |Pathways and Processes |Metabolic signaling pathways |Lipid and lipoprotein metabolism | |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Z-farnesyl diphosphate synthase (FDPS) is an enzyme belonging to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. Z-farnesyl diphosphate synthase (FDPS) functions as key enzyme in isoprenoid biosynthesis which catalyzes the formation of farnesyl diphosphate, a precurcor for several classes of essential metabolites. FDPS catalyzes the production of geranyl pyrophosphate and farnesyl pyrophosphate from isopentenyl pyrophosphate and dimethylallyl pyrophosphate. The resulting product, farnesyl pyrophosphate, is a key intermediate in cholesterol and sterol biosynthesis, a substrate for protein farnesylation and geranylgeranylation, and a ligand or agonist for certain hormone receptors and growth receptors. Drugs that inhibit this enzyme prevent the post-translational modifications of small GTPases and have been used to treat diseases related to bone resorption. Functions of FDPS may be inactivated by interferon-induced RSAD2. This inactivation may result of disruption of lipid rafts at the plasma membrane, and thus have an antiviral effect since many enveloped viruses need lipid rafts to bud efficiently out of the cell. |
| Reference |
