Human FKBP12 Protein (His Tag)
FKBP-12,FKBP-1A,FKBP1,FKBP12,PKC12,PKCI2,PPIASE
- 100ug (NPP3867) Please inquiry
| Catalog Number | P10268-H08E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | FKBP-12,FKBP-1A,FKBP1,FKBP12,PKC12,PKCI2,PPIASE |
| Molecular Weight | The recombinant human FKBP12 consists of 114 amino acids and predicts a molecular mass of 12.9 kDa as estimated by SDS-PAGE. |
| predicted N | Met 1 |
| SDS-PAGE | 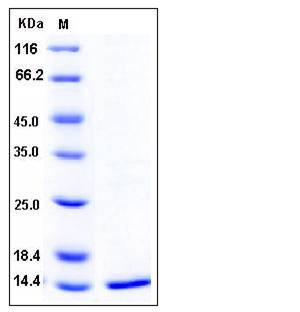 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human FKBP12 (NP_463460) (Met 1-Glu 108) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Phosphatase & Regulator |Phosphatase Regulator |
| Formulation | Lyophilized from sterile PBS, 10% glycerol, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | FK506 binding protein 12 (FKBP12), also known as FKBP1, along with cyclophilin, are two major members of the immunophilin protein family who serve as receptors for the immunosuppressant drugs cyclosporin A and FK506. As a conserved molecules in many eukaryotes, FKBP12 has been characterized as a peptidyl-prolyl isomerase that catalyzes the transition between cis- and trans-proline residues, and is involved in several biochemical processes including protein folding, receptor signaling, protein trafficking and transcription. FKBP12 has attracted immense attention and its role in mediating the immunosuppressive functions. FKBP12 serves a dual role as a peptidyl-prolyl cis-trans isomerase and as a modulator of several cell signaling pathways. In one such a role, FKBP12 interacts with and regulates the functional state of the ryanodine Ca2+ channel receptor by altering protein conformation and coordinating multi-protein complex formation. Another physiological role of FKBP12 is an interactor and a regulator of the type I serine/threonine kinase receptors of TGF-beta superfamily. Current data, derived from detailed biochemical studies as well as from functional studies in various systems, suggest that FKBP12 functions as a "guardian" for the type I receptors to prevent them from leaky signaling under sub-optimal ligand concentrations, thereby providing a molecular "gradient reader" for TGF-beta family morphogens. This aspect of FKBP12 function may be critical for cellular responsiveness to morphogenetic gradients of the TGF-beta family members during early development, serving to assure the translation of different ligand concentrations into different signaling readouts. In addition, FKBP12 may be involved in neuronal or astrocytic cytoskeletal organization and in the abnormal metabolism of tau protein in Alzheimer's disease (AD) damaged neurons. |
| Reference |
