Human FTL / ferritin, light polypeptide Protein (His Tag)
LFTD,MGC71996,NBIA3
- 100ug (NPP2136) Please inquiry
| Catalog Number | P12482-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | LFTD,MGC71996,NBIA3 |
| Molecular Weight | The recombinant human FTL consisting of 191 amino acids and has a calculated molecular mass of 22.1 kDa. It migrates as an approximately 23 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 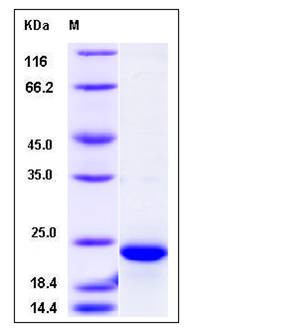 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human FTL (P02792) (Met 1-Asp 175) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Developmental Biology |Metabolism |Types of disease |Metabolism in Cancer |
| Formulation | Lyophilized from sterile 50mM Tris, 20% glycerol, pH 9.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ferritin, light polypeptide (FTL) is the light subunit of the ferritin protein. Ferritin is the major intracellular iron storage protein in prokaryotes and eukaryotes. It is composed of 24 subunits of the heavy and light ferritin chains. Storage of iron in the tissues occurs in the form of ferritin and hemosiderin. The latter originates from ferritin that has undergone intracellular digestion of its protein shell, leaving the iron core. Ferritin and hemosiderin are components of a continuum. Ferritin has been identified in all types of living organisms: animals, plants, molds, and bacteria. Whithin the protein shell of ferritin, iron is first oxidized to the ferric state for storage as ferric oxyhdroxide. Thus, ferritin removes excess iron from the cell sap where it could otherwise participate in peroxidation mechanisms. |
| Reference |
