Human FUOM / Fucose mutarotase / FucM / C10orf125 Protein (His Tag)
C10orf125,FucM,FUCU
- 100ug (NPP2139) Please inquiry
| Catalog Number | P13974-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | C10orf125,FucM,FUCU |
| Molecular Weight | The recombinant human C10orf125 consists of 169 amino acids and predicts a molecular mass of 18.6 KDa. It migrates as an approximately 19 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 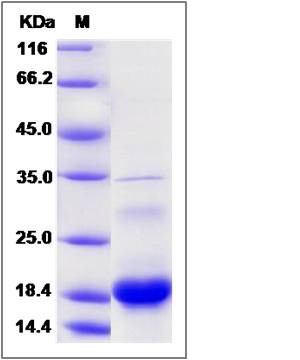 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human C10orf125 (A2VDF0-1) (Met1-Leu154) was expressed with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Metabolism |Pathways and Processes |Metabolic signaling pathways |Carbohydrate metabolism | |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | FUOM, also known as fucose mutarotase and FucM, belongs to the RbsD / FucU family. FUOM is involved in the interconversion between alpha- and beta-L-fucoses. L-Fucose has two isforms: alpha-L-fucose (29.5%) and beta-L-fucose (70.5%). The beta-form is metabolized through the salvage pathway. GDP-L-fucose formed either by the de novo or salvage pathways is transported into the endoplasmic reticulum, where it serves as a substrate for N- and O-glycosylations by fucosyltransferases. Fucosylated structures expressed on cell surfaces or secreted in biological fluids are believed to play a critical role in cell-cell adhesion and recognition processes. FUOM mainly exists as homodimer, but also functions as homotetramer, homooctamer, and homodecamer. FUOM's homodimeric form seems catalytically inactive. |
| Reference |
