Human GOPC / PIST Protein (His Tag)
CAL,dJ94G16.2,FIG,GOPC1,PIST
- 100ug (NPP3899) Please inquiry
| Catalog Number | P14911-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CAL,dJ94G16.2,FIG,GOPC1,PIST |
| Molecular Weight | The recombinant human GOPC consists of 192 amino acids and predicts a molecular mass of 21.1 KDa. It migrates as an approximately 25-30 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 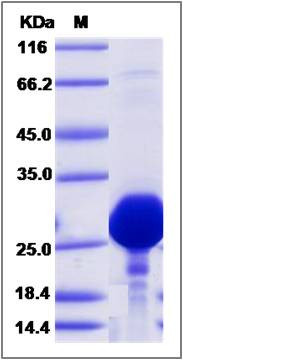 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human GOPC (Q9HD26-1) (Ile286-Tyr462) was expressed with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Protein Trafficking |Golgi Proteins |
| Formulation | Lyophilized from sterile PBS, 10% Glycerol, pH 7.4. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | GOPC, also known as PIST, interacts specifically with TC10 (a Rho-family small GTPase)] as a binding partner for Rhotekin. Rhotekin associates with PIST in vitro and in both polarized and non-polarized MDCK (Madin-Darby canine kidney) cells. The C-terminal SPV (Ser-Pro-Val) motif of Rhotekin binds to the PDZ domain of PIST. The co-localization of PIST and Rhotekin at the Golgi apparatus can be detected in non-polarized fibroblast-like MDCK cells and AJs (adherens junctions) in the fully polarized cells. PIST and Rhotekin are recruited from the cytosol to AJs as the cell becomes polarized. Expression of constitutively active Rho or prevention of Rhotekin-PIST interaction induced diffuse cytoplasmic distribution of Rhotekin in polarized MDCK cells. GOPC may regulate CFTR chloride currents and acid-induced ACCN3 currents by modulating cell surface expression of both channels. It may also regulate the intracellular trafficking of the ADR1B receptor. GOPC is ubiquitously expressed and its overexpression results in CFTR intracellular retention and degradation in the lysosomes. |
| Reference |
