Human GPHA2 / Glycoprotein hormone alpha 2 Protein (Fc Tag)
A2,GPA2,ZSIG51
- 100ug (NPP3904) Please inquiry
| Catalog Number | P13894-H05H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | A2,GPA2,ZSIG51 |
| Molecular Weight | The recombinant human GPHA2/mFc is a disulfide-linked homodimer. The reduced monomer comprises 340 amino acids and has a predicted molecular mass of 38.1 kDa. The apparent molecular mass of the protein is approximately 38-43 in SDS-PAGE under reducing conditions. |
| predicted N | Gln 24 |
| SDS-PAGE | 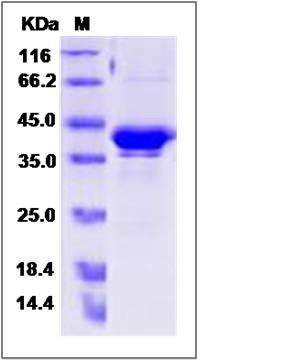 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human GPHA2 (Q96T91) (Met1-Tyr129) was fused with Fc region of mouse IgG at the C-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | GPHA2 is a member of the glycoprotein hormones subunit alpha family. Glycoprotein hormones consist of two subunits, the common alpha- and specific beta-subunits, which associate noncovalently to form a heterodimer. The alpha-subunit combines with four distinct beta-subunits giving rise to four biologically active hormones in human: FSH, LH, TSH, and CG. GPHA2 and glycoprotein hormone beta 5 (GPHB5) can form a noncovalent heterodimer. GPHA2 can be detected in a variety of tissues. Recombinant A2/B5 heterodimeric glycoproteins activates human TSH receptors, but not LH and FSH receptors, and shows high affinity to TSH receptors in a radioligand receptor assay. The heterodimer also stimulates cAMP production and thymidine incorporation by cultured thyroid cells and increases serum thyroxine levels in TSH-suppressed rats in vivo. This new heterodimeric glycoprotein hormone was named as thyrostimulin based on its thyroid-stimulating activity. The expression of thyrostimulin in the anterior pituitary known to express TSH receptors suggested a paracrine mechanism. |
| Reference |
