Human GSTK1 Protein
GST,GST13,GST13-13,GSTK1-1,hGSTK1
- 100ug (NPP2172) Please inquiry
| Catalog Number | P14291-HNAE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | GST,GST13,GST13-13,GSTK1-1,hGSTK1 |
| Molecular Weight | The recombinant human GSTK1 consists of 226 amino acids and predicts a molecular mass of 25.5 KDa. It migrates as an approximately 25 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 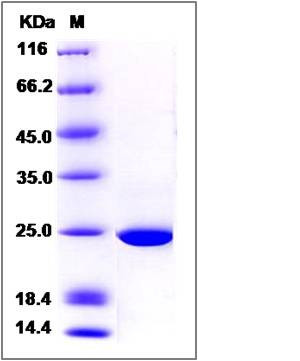 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human GSTK1 (Q9Y2Q3-1) (Gly2-Leu226) was expressed with a N-terminal Met. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Metabolism |Drug metabolism |
| Formulation | Lyophilized from sterile 50mM Tris,10% glycerol, pH 8.0. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | GSTK1 gene encodes a member of the kappa class of the glutathione transferase superfamily of enzymes that function in cellular detoxification. Glutathione S-transferases (GSTs) are a family of enzymes that catalyze a variety of reactions in both eukaryotes and prokaryotes. They catalyze the conjugation of reduced glutathione with potentially toxic, xenobiotic substrates, thus aiding excretion from the body. GSTK1(glutathione S-transferase kappa 1) is localized to the peroxisome and catalyzes the conjugation of glutathione to a wide range of hydrophobic substates facilitating the removal of these compounds from cells. GSTK1 functions in cellular detoxification. |
| Reference |
