Human Galectin-7 / LGALS7 Protein (GST Tag)
GAL7,LGALS7A
- 100ug (NPP2304) Please inquiry
| Catalog Number | P12000-H09E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | GAL7,LGALS7A |
| Molecular Weight | The recombinant human LGALS7/GST chimera consists of 366 amino acids and has a predicted molecular mass of 41.8 kDa. It migrates as an approxiamtely 40 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 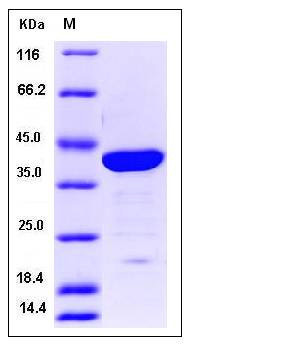 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human LGALS7 (P47929) (Ser 2-Phe 136) was fused with the GST tag at the N-terminus. |
| Bio-activity | Measured by its ability to agglutinate human red blood cells. The ED50 for this effect is typically 0.01-0.05 μg/mL. |
| Research Area | Cancer |Invasion microenvironment |Adhesion molecule |Cell adhesion |Lectin |Galectin | |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | LGALS7, also known as Galectin-7, is a member of the galectins family. The galectins are a family of beta-galactoside-binding proteins. There are at least 14 identified members in this family. Galectins share similarities in the CRD (the carbohydrate recognition domain). They are synthesized as cytosolic proteins. Though localized principally in the cytoplasm and lacking a classical signal peptide, galectins can also be stimulated to secretion by non-classical pathways or alternatively targeted to the nucleus. Galectins are implicated in modulating cell-cell and cell-matrix interactions. LGALS7 contains 1 galectin domain and is mainly expressed in stratified squamous epithelium. Galectin-7 could be involved in cell-cell and/or cell-matrix interactions necessary for normal growth control. LGALS7 is a pro-apoptotic protein that functions intracellularly upstream of JNK activation and cytochrome c release. |
| Reference |
