Human H1F0 / Histone H1 Protein (His Tag)
CPN60,GROEL,H10,H1FV,HLD4,HSP60,HSP65,HSPD1,HuCHA60,SPG13
- 100ug (NPP2175) Please inquiry
| Catalog Number | P10920-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CPN60,GROEL,H10,H1FV,HLD4,HSP60,HSP65,HSPD1,HuCHA60,SPG13 |
| Molecular Weight | The recombinant human H1F0 consisting of 205 amino acids and has a calculated molecular mass of 22.4 kDa. It migrates as an approximately 27 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 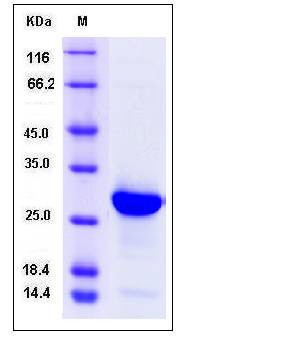 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human H1F0 (P07305) (Met 1-Lys 194) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Histone |H3 |Unmodified |
| Formulation | Lyophilized from sterile 50mM Tris, 600mM NaCl, 1mM DTT, pH 8.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | H1 histone family, member 0 (H1F0) is a member of the H1 histone family of nuclear proteins which are a component of chromatin in eukaryotic cells. It's involved in maintaining the structure of chromatin by packing the "beads on a string" sub-structure into a high order structure. The lysine-rich H1 histone family in mammals includes eleven members. In higher eukaryotes all H1 variants have the same general structure, consisting of a central conserved globular domain and less conserved N-terminal and C-terminal tails. These tails are moderately conserved among species, but differ among variants, suggesting a specific function for each H1 variant. Studies on the role of particular subtypes at specific developmental stages in lower eukaryotes, but also in vertebrates suggest that specific subtypes of H1 participate in particular systems of gene regulation. |
| Reference |
