Human HAO1 / GOX1 Protein (His Tag)
GOX,GOX1,HAOX1
- 100ug (NPP3914) Please inquiry
| Catalog Number | P12076-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | GOX,GOX1,HAOX1 |
| Molecular Weight | The recombinant human HAO1 consisting of 376 amino acids and migrates as an 42 kDa band in SDS-PAGE under reducing conditions as predicted. |
| predicted N | Met |
| SDS-PAGE | 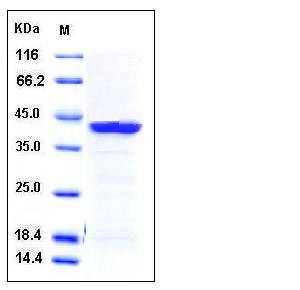 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the native human HAO1 (NP_060015.1) (Leu 2-Ile 370) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Heart |Apoptosis |Oxidative Stress |Additional Reduction/Oxidation (Redox) Enzymes |
| Formulation | Lyophilized from sterile 20mM Tirs, 500mM NaCl, 10% glycerol, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Hydroxyacid oxidase 1, also known as Glycolate oxidase, HAO1 and GOX1, is a member of the FMN-dependent alpha-hydroxy acid dehydrogenase family. HAO1 / GOX1 has 2-hydroxyacid oxidase activity. It is most active on the 2-carbon substrate glycolate, but is also active on 2-hydroxy fatty acids, with high activity towards 2-hydroxy palmitate and 2-hydroxy octanoate. HAO1 / GOX1 is a liver-specific peroxisomal enzyme that oxidizes glycolate to glyoxylate with concomitant production of H2O2. In Hao1 messenger RNA (mRNA), an iron-responsive element (IRE) homologous to the sequence recognized by iron regulatory proteins (IRP), key regulators of iron homeostasis, is present. Mammalian HAO1 / GOX1 is a peroxisomal protein and that the C-terminal sequence SKI acts as the targeting signal. Down-regulation of HAO1 / GOX1 expression during oxidative stress may provide a mechanism to prevent excessive H2O2 formation in liver peroxisomes and may represent the prototype of a poorly recognized but potentially relevant response to oxidative injury involving down-regulation of ROS-producing enzymes. |
| Reference |
