Human HDAC4 Protein (aa 612-1084)
AHO3,BDMR,HA6116,HD4,HDAC-4,HDAC-A,HDACA
- 100ug (NPP3917) Please inquiry
| Catalog Number | P11807-HNCB |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | AHO3,BDMR,HA6116,HD4,HDAC-4,HDAC-A,HDACA |
| Molecular Weight | The secreted recombinant human HDAC4 consists of 475 amino acids and predicts a molecular mass of 50.9 KDa. The apparent molecular mass of the protein is approximately 51 KDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Met |
| SDS-PAGE | 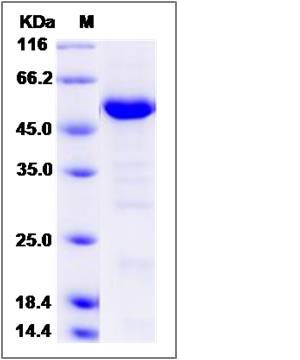 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human HDAC4 (Met612-Leu1084) was expressed and purified with two additional amino acids (Gly & Pro ) at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Signaling Pathway |Representative pathway |Notch Signaling pathway |
| Formulation | Lyophilized from sterile 20mM Tris,500mM NaCl, pH 7.4, 10%gly. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | HDAC4 (histone deacetylase 4), belongs to class II of the histone deacetylase/acuc/apha family. Histone Deacetylases (HDACs) are a group of enzymes closely related to sirtuins. They catalyze the removal of acetyl groups from lysine residues in histones and non-histone proteins, resulting in transcriptional repression. In general, they do not act autonomously but as components of large multiprotein complexes, such as pRb-E2F and mSin3A, that mediate important transcription regulatory pathways. There are three classes of HDACs; classes 1, 2 and 4, which are closely related Zn2+-dependent enzymes. HDACs are ubiquitously expressed and they can exist in the nucleus or cytosol. Their subcellular localization is effected by protein-protein interactions and by the class to which they belong. HDACs have a role in cell growth arrest, differentiation and death and this has led to substantial interest in HDAC inhibitors as possible antineoplastic agents. HDAC4 possesses histone deacetylase activity and represses transcription when tethered to a promoter. It does not bind DNA directly, but through transcription factors MEF2C and MEF2D. HDAC4 seems to interact in a multiprotein complex with RbAp48 and HDAC3. |
| Reference |
