Human HPRG / HRG Protein (His Tag)
HPRG,HRGP,THPH11
- 100ug (NPP3927) Please inquiry
| Catalog Number | P10836-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | HPRG,HRGP,THPH11 |
| Molecular Weight | The secreted recombinant human HPRG comprises 518 amino acids with a predicted molecular mass of 59 kDa. As a result of glycosylation, rhHPRG migrates as an approximately 75-80 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Val 19 |
| SDS-PAGE | 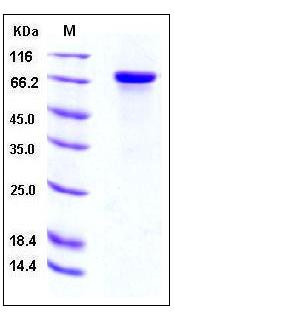 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human HPRG (NP_000403.1) (Met 1-Lys 525) with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | Measured by its ability to support the adhesion of MOLT-4 human acute lymphoblastic leukemia cells. (Lamb-Wharton, R.J. and W.T. Morgen, 1993, Cell Immunol. 152: 544) . Human HPRG immobilized at 1 μg/ml (100 μl/well) will induce >65% MOLT4 cell adhesion (1 x 10E5 cells/well) in the presence of 7.5 μg/ml Concanvalin A. |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Complement and Coagulation |Coagulation |Intrinsic |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Histidine-rich glycoprotein, also known as HRG and HPRG, is a glycoprotein located in plasma and platelets, and contains an unusually large amount of histidine and proline. In human, five distinct domains are recognized in the mature HPRG molecule. There are two N-terminal cystatin-like modules (aa 19 - 254) and one His-Pro-rich region (aa 350 - 497) that is flanked by two Pro-rich segments (aa 276 - 321 and 498 - 525). The His-Pro-rich region contains 10 tandem repeats with an HHPHG motif, and the N- and C-termini are linked by a disulfide bond. The specific functions of HRG remain unclear, but it is known that the protein binds heme, dyes and divalent metal ions. It inhibits rosette formation and interacts with heparin, thrombospondin and plasminogen. Two of the protein's effects, the inhibition of fibrinolysis and the reduction of inhibition of coagulation, indicate a potential prothrombotic effect. HPRG is evolutionarily, functionally and structurally related to cleaved high molecular weight kininogen (HKa), an anti-angiogenic polypeptide that stimulates apoptosis of proliferating endothelial cells through binding to cell-surface tropomyosin. The antiangiogenic activity of the multidomain plasma protein HPRG is localized to its histidine-proline-rich (H/P) domain and has recently been shown to be mediated, at least partially, through binding to cell-surface tropomyosin in fibroblast growth factor-2-activated endothelial cells. |
| Reference |
