Human IFI30 Protein (His Tag)
GILT,IFI-30,IFI30,IP-30,IP30
- 100ug (NPP3979) Please inquiry
| Catalog Number | P12623-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | GILT,IFI-30,IFI30,IP-30,IP30 |
| Molecular Weight | The recombinant human IFI30 is a disulfide-linked homodimer. The reduced monomer comprises 217 amino acids and has a predicted molecular mass of 24.7 kDa. The apparent molecular mass of the protein is approximately 31-34 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Ser 27 |
| SDS-PAGE | 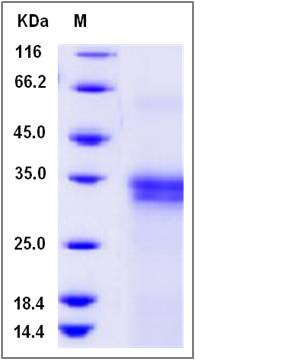 |
| Purity | > 96 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human IFI30 (P13284) (Met1-Lys243) with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Metabolism |Pathways and Processes |Redox metabolism |Oxidative stress | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | IFI30 belongs to the GILT family. This family includes the two characterised human gamma-interferon-inducible lysosomal thiol reductase (GILT) sequences: P13284 and Q9UL08. It also contains several other eukaryotic putative proteins with similarity to GILT. The aligned region contains three conserved cysteine residues. In addition, the two GILT sequences possess a C-X(2)-C motif that is shared by some of the other sequences in the family. This motif is thought to be associated with disulphide bond reduction. IFI30 is a lysosomal thiol reductase that can reduce protein disulfide bonds. It facilitates the generation of MHC class II-restricted epitodes from disulfide bond-containing antigen by the endocytic reduction of disulfide bonds. It also facilitates MHC class I-restricted recognition of exogenous antigens containing disulfide bonds by CD8+ T-cells or crosspresentation. IFI30 may facilitate the complete unfolding of proteins destined for lysosomal degradation and plays an important role in antigen processing. |
| Reference |
