Human IFNAR1 / IFNAR Protein (Fc Tag)
AVP,IFN-alpha-REC,IFNAR,IFNBR,IFRC
- 100ug (NPP2259) Please inquiry
| Catalog Number | P13222-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | AVP,IFN-alpha-REC,IFNAR,IFNBR,IFRC |
| Molecular Weight | The recombinant human IFNAR1/Fc is a disulfide-linked homodimeric protein. The reduced monomer consists of 650 amino acids and has a predicted molecular mass of 74 kDa. The apparent molecular mass of the reduced monomer is approximately 100-120 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Lys 28 |
| SDS-PAGE | 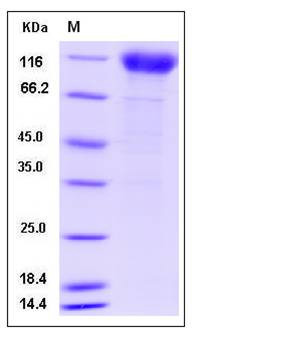 |
| Purity | > 88 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human IFNAR1 isoform 1 (P17181-1) extracellular domain (Met 1-Lys 436) was fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Angiogenesis |Cytokines / Chemokines in Angiogenesis |Interferons |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Interferon-alpha/beta receptor alpha chain (IFNAR1) is a type I membrane protein that forms one of the two chains of a receptor for interferons alpha and beta. Binding and activation of the receptor stimulates Janus protein kinases, which in turn phosphorylate several proteins, including STAT1 and STAT2. The encoded protein also functions as an antiviral factor. Tyk2 slows down IFNAR1 degradation and that this is due, at least in part, to inhibition of IFNAR1 endocytosis. Mutant versions of IFNAR1, in which Tyr466 is changed to phenylalanine, can act in a dominant negative manner to inhibit phosphorylation of STAT2. These observations are consistent with a model in which IFNAR1 mediates the interaction between JAK kinases and the STAT transcription factors. |
| Reference |
