Human IGJ / Immunoglobulin J chain Protein (His Tag)
IGCJ,JCH
- 100ug (NPP3990) Please inquiry
| Catalog Number | P13015-H08E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | IGCJ,JCH |
| Molecular Weight | The recombinant human IGJ comprises 147 amino acids and has a predicted molecular mass of 17 kDa. It migrates as an approximately 26 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Gln 23 |
| SDS-PAGE | 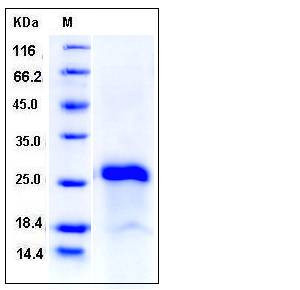 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the mature form of human IGJ (NP_653247.1) (Gln 23-Asp 159) was fused with a polyhistidine tag at the C-terminus and a pelB singnal peptide at the N-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Immunoglobulin J chain, also known as IGJ and IGCJ, is a secreted polypeptide which is the first immunoglobulin-related polypeptide expressed during the embryogenesis and differentiation of B cells in the fetal liver. The joining Immunoglobulin J chain is a small polypeptide, expressed by mucosal and glandular plasma cells, which regulates polymer formation of immunoglobulin (Ig)A and IgM. Immunoglobulin J chain / IGJ serves to link two monomer units of either IgM or IgA. In the case of IgM, the J chain-joined dimer is a nucleating unit for the IgM pentamer, and in the case of IgA it induces larger polymers. Immunoglobulin J chain / IGJ also help to bind these immunoglobulins to secretory component. J-chain incorporation into polymeric IgA (pIgA, mainly dimers) and pentameric IgM endows these antibodies with several salient features. Immunoglobulin J chain / IGJ is involved in creating the binding site for pIgR / SC in the Ig polymers, not only by determining the polymeric quaternary structure but apparently also by interacting directly with the receptor protein. Both the immunoglobulin J chain / IGJ and the pIgR/SC are key proteins in secretory immunity. |
| Reference |
