Human ITCH / AIP4 Protein (aa 526-903)
ADMFD,AIF4,AIP4,dJ468O1.1,NAPP1
- 100ug (NPP4030) Please inquiry
| Catalog Number | P11131-HNCE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | ADMFD,AIF4,AIP4,dJ468O1.1,NAPP1 |
| Molecular Weight | The recombinant human ITCH (aa 526-903) consisting of 380 amino acids and has a calculated molecular mass of 40 kDa. as estimated in SDS-PAGE under reducing conditions. |
| predicted N | Gly |
| SDS-PAGE | 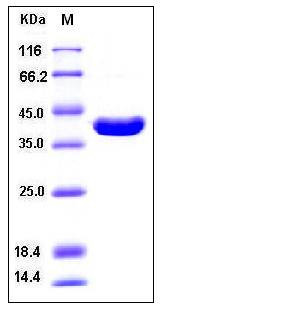 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human ITCH (NP_113671.3) N-terminal segment (Arg 526-Glu 903) was expressed and purified, with two additional amino acids (Gly & Pro) at the N-terminus. |
| Bio-activity | |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |RNA virus |+ssRNA virus | |
| Formulation | Lyophilized from sterile 20mM Tris, 200mM NaCl, 10% glycerol, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | E3 ubiquitin-protein ligase Itchy homolog, also known as Atrophin-1-interacting protein 4, NFE2-associated polypeptide 1, NAPP1 and ITCH, is a cell membrane protein which contains one C2 domain, one HECT (E6AP-type E3 ubiquitin-protein ligase) domain and contains four WW domains. ITCH acts as an E3 ubiquitin-protein ligase which accepts ubiquitin from an E2 ubiquitin-conjugating enzyme in the form of a thioester and then directly transfers the ubiquitin to targeted substrates. It catalyzes 'Lys-29'-, 'Lys-48'- and 'Lys-63'-linked ubiquitin conjugation. ITCH is involved in the control of inflammatory signaling pathways. It is an essential component of a ubiquitin-editing protein complex, comprising also TNFAIP3, TAX1BP1 and RNF11, that ensures the transient nature of inflammatory signaling pathways. ITCH promotes the association of the complex after TNF stimulation. Once the complex is formed, TNFAIP3 deubiquitinates 'Lys-63' polyubiquitin chains on RIPK1 and catalyzes the formation of 'Lys-48'-polyubiquitin chains. This leads to RIPK1 proteosomal degradation and consequently termination of the TNF- or LPS-mediated activation of NFKB1. Defects in ITCH are the cause of syndromic multisystem autoimmune disease (SMAD) which is characterized by organomegaly, failure to thrive, developmental delay, dysmorphic features and autoimmune inflammatory cell infiltration of the lungs, liver and gut. |
| Reference |
