Human IgG1-Fc Protein (103 Cys/Ser)
IgG1 Fc,Ighg1
- 100ug (NPP3988) Please inquiry
| Catalog Number | P10702-HNAH |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | IgG1 Fc,Ighg1 |
| Molecular Weight | The recombinant human IgG1 Fc consists of 232 amino acids and has a predicted molecular mass of 26 kDa. As a result of glycosylation, the apparent molecular mass of rhFc is approximately 32 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Glu 99 |
| SDS-PAGE | 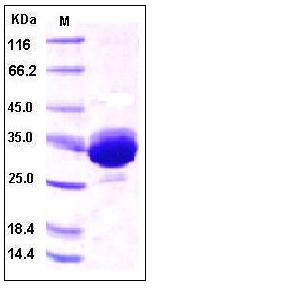 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human IgG1 Fc region (AAC82527.1) (Glu 99-Lys 330) (one aa mutation,103 Cys/Ser) was expressed. |
| Bio-activity | 1. Measured by its ability to bind human CD16a-His (P10389-H08H1) in a functional ELISA. 2. Measured by its ability to bind human CD16a-AVI-His (P10389-H27H) in a functional ELISA. 3.Measured by its ability to bind human CD16a-His (P10389-H08H) in a functional ELISA. |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | As a monomeric immunoglobulin that is predominately involved in the secondary antibody response and the only isotype that can pass through the human placenta, Immunoglobulin G (IgG) is synthesized and secreted by plasma B cells, and constitutes 75% of serum immunoglobulins in humans. IgG antibodies protect the body against the pathogens by agglutination and immobilization, complement activation, toxin neutralization, as well as the antibody-dependent cell-mediated cytotoxicity (ADCC). IgG tetramer contains two heavy chains (50 kDa ) and two light chains (25 kDa) linked by disulfide bonds, that is the two identical halves form the Y-like shape. IgG is digested by pepsin proteolysis into Fab fragment (antigen-binding fragment) and Fc fragment ("crystallizable" fragment). IgG1 is most abundant in serum among the four IgG subclasses (IgG1, 2, 3 and 4) and binds to Fc receptors (FcγR ) on phagocytic cells with high affinity. Fc fragment is demonstrated to mediate phagocytosis, trigger inflammation, and target Ig to particular tissues. Protein G or Protein A on the surface of certain Staphylococcal and Streptococcal strains specifically binds with the Fc region of IgGs, and has numerous applications in biotechnology as a reagent for affinity purification. Recombinant IgG Fc Region is suggested to represent a potential anti-inflammatory drug for treatment of human autoimmune diseases. |
| Reference |
