| Catalog Number |
P10734-H07H |
| Organism Species |
Human |
| Host |
Human Cells |
| Synonyms |
FLJ23633,FLJ23701,FLJ23807,L-RAP,LRAP,LRAP.ERAP2 |
| Molecular Weight |
The recombinant human ERAP2 consists of 921 amino acids and predictes a molecular mass of 106 kDa. In SDS-PAGE under reducing conditions, it migrates with the apparent molecular mass of 115-125 kDa due to glycosylation. |
| predicted N |
Leu 24 |
| SDS-PAGE |
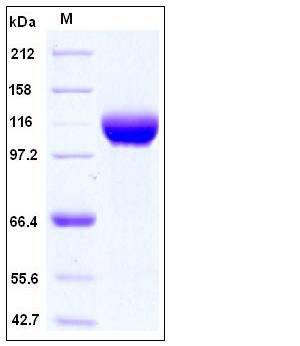 |
| Purity |
> 98 % as determined by SDS-PAGE |
| Protein Construction |
A DNA sequence encoding the lumenal domain of human ERAP2 (NP_071745.1) (Ala 56-Thr 960) was expressed with a polyhistidine tag at the N-terminus. |
| Bio-activity |
Measured by its ability to cleave the fluorogenic peptide substrate, Arg-7-amido-4-methylcoumarin (Arg-AMC). The specific activity is >50 pmoles/min/μg. |
| Research Area |
Immunology |Adaptive Immunity |Major Histocompatibility Complex (MHC) |MHC Class I |
| Formulation |
Lyophilized from sterile 1 |
| Background |
Leukocyte-derived arginine aminopeptidase (LRAP), also known as endoplasmic reticulum-aminopeptidase 2 (ERAP2), is the second identified aminopeptidase localized in the in the lumenal side of endoplasmic reticulum (ER) processing antigenic peptides presented to major histocompatibility complex (MHC) class I molecules. It is a 960-amino acid protein with significant homology to placental leucine aminopeptidase and adipocyte-derived leucine aminopeptidase. LRAP preferentially hydrolyzes the basic residues Arg and Lys, and contains the HEXXH(X)18E zinc-binding motif, which is the characteristic of the M1 family of zinc metallopeptidases which also includes PILS/ARTS1/ERAP1 and LNPEP/PLAP. Induced by interferon-gamma, LRAP is able to trim various MHC class I antigenic peptide precursors. |
| Reference |
|

