Human METTL11A Protein
AD-003,C9orf32,HOMT1A,METTL11A,NRMT,NTM1A
- 100ug (NPP4082) Please inquiry
| Catalog Number | P11222-HNCE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | AD-003,C9orf32,HOMT1A,METTL11A,NRMT,NTM1A |
| Molecular Weight | The recombinant human METTL11A consists of 224 amino acids and has a predicted molecular mass of 25.5 kDa as estimated by SDS-PAGE under reducing conditions. |
| predicted N | Gly |
| SDS-PAGE | 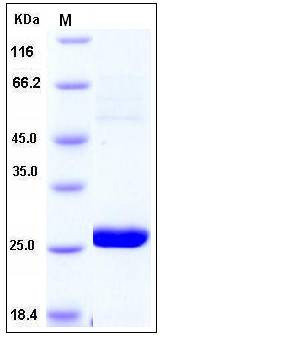 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human METTL11A (NP_054783.2) (Thr 2-Arg 223) was expressed and purified, with additional two amino acids (Gly & Pro) at the N-terminus. |
| Bio-activity | |
| Research Area | Microbiology |Pathogenic microorganism |viruses |animal virus |Virus antigen |DcCoV viral antigen | |
| Formulation | Lyophilized from sterile 20mM Tris, 150mM NaCl, 10% glycerol, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Methyltransferase-like protein 11A, also known as METTL11A, is a member of the methyltransferase superfamily and METTL11 family. Methyltransferase is a type of transferase enzyme which transfers a methyl group from a donor to an acceptor. Methylation often occurs on nucleic bases in DNA or amino acids in protein structures. Methytransferase uses a reactive methyl group bound to sulfur in S-adenosyl methionine (SAM) as the methyl donor. DNA methylation is often utilized to silence and regulate genes without changing the original DNA sequence. This methylation occurs on cytosine residues. DNA methylation may be necessary for normal growth from embryonic stages in mammals. Methylation can serve to protect DNA from enzymatic cleavage, since restriction enzymes are unable to bind and recognize externally modified sequences. This is especially useful in bacterial restriction modification systems which use restriction enzymes to cleave foreign DNA while keeping their own DNA protected by methylation. Methylation of amino acids in the formation of proteins leads to more diversity of possible amino acids and therefore more diversity of function. The methylation reaction occurs on nitrogen atoms either on the N terminus or side-chain position of the protein and are usually irreversible. |
| Reference |
