Human MFAP5 / MAGP2 Protein (His Tag)
AAT9,MAGP-2,MAGP2,MFAP-5,MP25
- 100ug (NPP2341) Please inquiry
| Catalog Number | P12648-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | AAT9,MAGP-2,MAGP2,MFAP-5,MP25 |
| Molecular Weight | The recombinant human MFAP5 comprises 163 amino acids and has a predicted molecular mass of 18.7 kDa. The apparent molecular mass of the protein is approximately 54 and 29 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Arg 22 |
| SDS-PAGE | 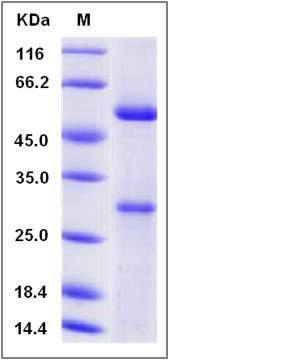 |
| Purity | > (54.7+31.5) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human MFAP5 (Q13361) (Met1-Leu173) with a C-terminal polyhistidine tag was expressed. |
| Bio-activity | |
| Research Area | Cardiovascular |Angiogenesis |Angiogenesis Growth Factor & Receptor |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | MFAP5, also known as MAGP2, is a component of the elastin-associated microfibrils. It belongs to the MFAP family. As a 25-kD microfibril-associated glycoprotein, MFAP5 is rich in serine and threonine residues. It stimulates the assembly of elastic fibers, a complex structure composed of a tropoelastin inner core and microfibril outer mantle comprising proteins such as fibrillins and microfibril-associated glycoproteins that guide tropoelastin deposition. MFAP5 also stabilizes type 1 procollagen and thus plays an important role in extracellular matrix homeostasis. It has multiple binding regions on fibrillins and has covalent periodic association with fibrillin-containing microfibrils. |
| Reference |
