Human MMP-2 / CLG4A Protein
CLG4,CLG4A,MMP-2,MMP-II,MONA,TBE-1
- 100ug (NPP4085) Please inquiry
| Catalog Number | P10082-HNAH |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CLG4,CLG4A,MMP-2,MMP-II,MONA,TBE-1 |
| Molecular Weight | The recombinant human MMP2 consists of 631 amino acids and migrates as an 72 kDa band as predicted in SDS-PAGE under reducing conditions. |
| predicted N | Ala 30 |
| SDS-PAGE | 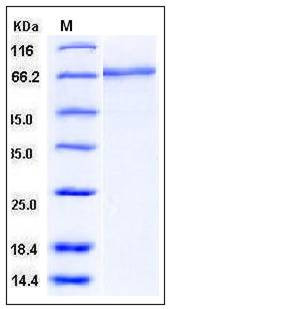 |
| Purity | > (74.7+17.3) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the native human MMP2 (NP_004521.1) (Met 1-Cys 660) was expressed and purified. |
| Bio-activity | 1. Measured by its ability to cleave the fluorogenic peptide substrate Mca-PLGL-Dpa-AR-NH2 (AnaSpec, Catalog # 27076). The specific activity is > 1,000 pmoles/min/µg. 2. Measured by its binding ability in a functional ELISA. 3. Immobilized human MMP2 at 10 μg/mL (100 μl/well) can bind human TIMP2/Fc (P 10396-H01H). The EC50 of human TIMP2/Fc is 0.02 μg/mL. (Activation description: The proenzyme needs to be activated by APMA for an activated form) |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Inflammatory Disorders Therapeutic Targets |Rheumatoid arthritis Therapeutic Targets |
| Formulation | Lyophilized from sterile 0.05 % Brij-35, 150 mM NaCl, 5 mM CaCl2, 50 mM Tris, pH 7.5. 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Matrix Metalloproteinase-2 (MMP-2) is an enzyme that degrades components of the extracellular matrix and thus plays a pivotal role in cell migration during physiological and pathological processes. MMP-2 expression is dependent on extracellular matrix metalloproteinase inducer (EMMPRIN), Her2/neu, growth factors, cytokines, and hormones. Pro-MMP-2 activation needs MT1-MMP and TIMP-2 contribution. MMP-2 is changed in distribution and increased in amount in the ventral cochlear nucleus after unilateral cochlear ablation. A low level of MMP-2 is linked to favorable prognosis in patients with a hormone receptor-negative tumor, usually associated with high risk. As a zymogen requiring proteolytic activation for catalytic activity, MMP-2 has been implicated broadly in the invasion and metastasis of many cancer model systems, including human breast cancer (HBC). Blocking MMP-2 secretion and activation during breast carcinoma development may decrease metastasis. The detection of active MMP-2 alone or the rate of pro-MMP-2 and active MMP-2 is considered a very sensitive indicator of cancer metastasis. Modulation of MMP-2 expression and activation through specific inhibitors and activators may thus provide a new mechanism for breast cancer treatment. |
| Reference |
