Human MMP-9 / CLG4B Protein
CLG4B,GELB,MANDP2,MMP-9
- 100ug (NPP4090) Please inquiry
| Catalog Number | P10327-HNAH |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CLG4B,GELB,MANDP2,MMP-9 |
| Molecular Weight | The recombinant human MMP9 consisting of 688 amino acids has a predicted molecular mass of 76.3 kDa. The apparent molecular mass of rh MMP9 is approximately 80-95 kDa in SDS-PAGE due to glycosylation. |
| predicted N | Ala 20 |
| SDS-PAGE | 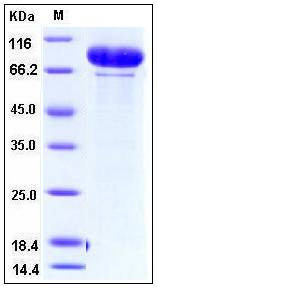 |
| Purity | > 94 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human MMP9 zymogen (NP_004985.2) (Met 1-Asp 707) was expressed. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, Mca-PLGL-Dpa-AR-NH2, (AnaSpec, Cat#27076). The specific activity is >600 pmoles/min/μg . |
| Research Area | Cardiovascular |Blood |Coagulation |Common |
| Formulation | Lyophilized from sterile 50mM Tris, 0.15M NaCl, 0.01MCaCl2, 0.05% Brig-35, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Matrix metalloproteinases (MMPs) are neutral proteinases that are involved in the breakdown and remodeling of the extracellular matrix (ECM) under a variety of physiological and pathological conditions, such as morphogenesis, differentiation, angiogenesis and tissue remodeling, as well as pathological processes including inflammation, arthritis, cardiovascular diseases, pulmonary diseases and tumor invasion. MMP9, also known as 92-kDa gelatinase B/type IV collagenase, is secreted from neutrophils, macrophages, and a number of transformed cells, and is the most complex family member in terms of domain structure and regulation of its activity. It plays an important role in tissue remodelling in normal and pathological inflammatory processes. MMP-9 is a major secretion product of macrophages and a component of cytoplasmic granules of neutrophils, and is particularly important in the pathogenesis of inflammatory, infectious, and neoplastic diseases in many organs including the lung. This enzyme is also secreted by lymphocytes and stromal cells upon stimulation by inflammatory cytokines, or upon delivery of bi-directional activation signals following integrin-mediated cell-cell or cell-extracellular matrix (ECM) contacts. Since the integrity of the tissue architecture is closely dependent of the delicate balance between MMPs and their inhibitors, excessive production of MMP-9 is linked to tissue damage and degenerative inflammatory disorders. As a consequence, regulation of gene transcription and tissue-specific expression of MMP-9 in normal and diseased states are being actively investigated to pave the way for new therapeutic targets. In addition, the dramatic overexpression of MMP-9 in cancer and various inflammatory conditions clearly points to the molecular mechanisms controlling its expression as a potential target for eventual rational therapeutic intervention. |
| Reference |
