Human MMP12 / MMP-12 / HME Protein (catalytic domain)
HME,ME,MME,MMP-12
- 100ug (NPP4092) Please inquiry
| Catalog Number | P10266-HNAE |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | HME,ME,MME,MMP-12 |
| Molecular Weight | The recombinant human MMP12 consists of 164 amino acids and predicts a molecular mass of 18.2 KDa. It migrates as an approximately 18 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 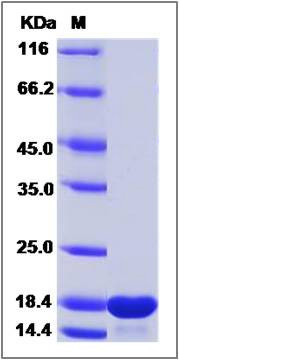 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding human MMP12 (NP_002417.2) (Gly106-Asn268) was expressed with a N-terminal Met. |
| Bio-activity | Measured by its ability to cleave the fluorogenic peptide substrate, Mca-PLGL-Dpa-AR-NH2 (AnaSpec, Catalog # 27076). The specific activity is > 800 pmoles/min/µg. |
| Research Area | Cardiovascular |Atherosclerosis |Thrombosis |Other in Thrombosis |
| Formulation | Lyophilized from sterile 10 mM Hepes, 2 mM CaCl2, 250 mM NaCl, pH 7.0. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrade components of the extracellular matrix (ECM) and play essential roles in various physiological processes such as morphogenesis, differentiation, angiogenesis and tissue remodeling, as well as pathological processes including inflammation, arthritis, cardiovascular diseases, pulmonary diseases and tumor invasion. Macrophage metalloelastase, also known as Matrix metalloproteinase-12, Macrophage elastase, MMP12, and MMP-12, is a secreted protein which belongs to the peptidase M10A family. MMP12 is a macrophage-secreted elastase that is highly induced in the liver and lung in response to S. mansoni eggs and contains four hemopexin-like domains. MMP12 is a proteolytic enzyme responsible for cleavage of plasminogen to angiotensin, which has an angiostatic effect. It may be involved in tissue injury and remodeling and has significant elastolytic activity. It may be related to prognosis in breast cancer patients. MMP12 promotes fibrosis by limiting the expression of specific ECM-degrading MMPs. Like MMP12, MMP13 expression is highly dependent on IL-13 and type I I-IL-4 receptor signaling. MMP12 is a potent proinflammatory and oncogenic molecule. MMP12 up-regulation plays a critical role in emphysema to lung cancer transition that is facilitated by inflammation. |
| Reference |
