Human MMP7 Protein (Fc Tag)
MMP-7,MPSL1,PUMP-1
- 100ug (NPP4087) Please inquiry
| Catalog Number | P10277-H01H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | MMP-7,MPSL1,PUMP-1 |
| Molecular Weight | The recombinant human Fc/MMP7 chimera is a disulfide-linked homodimeric protein. The reduced monomer consists of 410 amino acids and predicts a molecular mass of 45.8 kDa. As a result of glycosylation, the rh Fc/MMP7 monomer migrates as an approximately 55 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Glu 20 |
| SDS-PAGE | 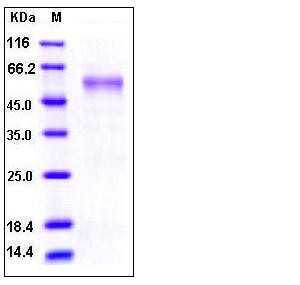 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the active form of human MMP7 (NP_002414.1) (Tyr 95-Lys 267) was expressed with the fused Fc region of human IgG1 at the N-terminus. |
| Bio-activity | |
| Research Area | Cardiovascular |Atherosclerosis |Vascular Inflammation |Leukocyte recruitment |Other in Leukocyte recruitment |
| Formulation | Lyophilized from sterile 100mM Glycine, 10mM NaCl, 50mM Tris, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Matrix metalloproteinases (MMPs) are a family of zinc-dependent endopeptidases that degrade components of the extracellular matrix (ECM) and play essential roles in various physiological and pathological processes such as morphogenesis, differentiation, angiogenesis, tissue remodeling, and tumor invasion. MMPs are synthesized as pro-enzymes and converted to active form by extracellular proteinases. MMP7, also referred to as matrilysin, is the smallest member of the MMP family and differs from other MMP members in that it lacks the C-terminal hemopexin-like domain. MMP7 is produced primarily by mucosal epithelia, and is capable of degrading various ECM proteins including proteoglycans, fibronectin, elastin and casein. This enzyme serves essential functions in both innate defense and wound healing, and appears to be one of the most important MMPs in human colon cancers. It has been reported that MMP7 contributes to tumor malignancy probably by cleaving cell surface proteins such as Fas ligand, degradation of IgG or inducing E-cadherin-mediated cell aggregation. In addition, matrilysin is also identified as a mediator of pulmonary fibrosis and a potential therapeutic target. |
| Reference |
