Human MOBKL1A / MOB1B Protein (GST Tag)
MATS2,MOB4A,MOBKL1A
- 100ug (NPP4093) Please inquiry
| Catalog Number | P14016-H09E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | MATS2,MOB4A,MOBKL1A |
| Molecular Weight | The recombinant human MOBKL1A /GST chimera consists of 450 amino acids and has a predicted molecular mass of 52.3 kDa. It migrates as an approximately 43-48 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 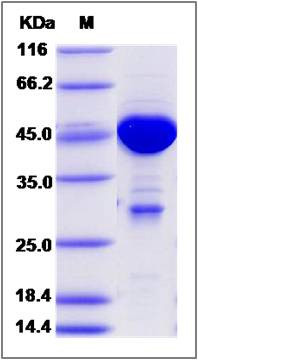 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human MOBKL1A (Q7L9L4) (Met1-Arg216) was fused with the GST tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |DNA / RNA |RNA Processing |Other in RNA processing |
| Formulation | Lyophilized from sterile 20mM Tris, 150mM NaCl, pH 8.0. 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | MST1 and MST2 are the mammalian Ste20-related protein kinases most closely related to Drosophila Hippo, a major regulator of cell proliferation and survival during development. Overexpression of MST1 or MST2 in mammalian cells is proapototic. MST1 and MST2 activity increases during mitosis, especially in nocodazole-arrested mitotic cells, where these kinases exhibit both an increase in both abundance and activation. MST1 and MST2 also can be activated nonphysiologically by okadaic acid or H2O2. The MOB1B and MOBKL1B polypeptides, homologs of the Drosophila MATS polypeptide, are identified as preferred MST1/MST2 substrates in vitro and are phosphorylated in cells in an MST1/MST2-dependent manner in mitosis and in response to okadaic acid or H2O2. MST1/MST2-catalyzed MOB1B/MOBKL1B phosphorylation alters the ability of MOB1B/MOBKL1B to bind and regulate downstream targets such as the NDR-family protein kinases. Thus, MOB1B/MOBKL1B phosphorylation in cells promotes MOB1B/MOBKL1B binding to the LATS1 kinase and enables H2O2-stimulated LATS1 activation loop phosphorylation. Most importantly, replacement of endogenous MOB1B/MOBKL1B by a nonphosphorylatable mutant is sufficient to accelerate cell proliferation substantially by speeding progression through G1/S as well as mitotic exit. |
| Reference |
