Human MSN / Moesin Protein (aa 1-346, His Tag)
HEL70
- 100ug (NPP2352) Please inquiry
| Catalog Number | P13659-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | HEL70 |
| Molecular Weight | The recombinant human MSN consists of 361 amino acids and has a calculated molecular mass of 42.8 kDa. It migrates as an approximately 45 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 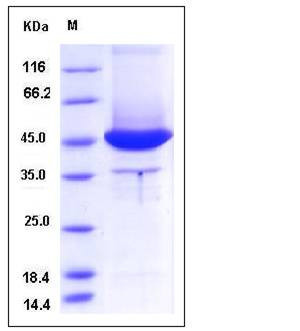 |
| Purity | > 80 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human MSN (P26038) (Met 1-Glu 346) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Cytoskeleton / ECM |Cytoskeletal Proteins |Microfilaments |Talin / Moesin | |
| Formulation | Lyophilized from sterile 20mM Tris, 0.5M NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Moesin is a member of the ERM family which includes ezrin and radixin. ERM proteins, highly related members of the larger protein 4.1 superfamily, can exist in an active or inactive conformation. It seems that ERM proteins function as cross-linkers between plasma membranes and actin-based cytoskeletons. The sole Drosophila ERM protein, moesin, functions to promote cortical actin assembly and apical-basal polarity. As a result, cells lacking moesin lose epithelial characteristics and adopt invasive migratory behaviour. It is localized to filopodia and other membranous protrusions that are important for cell-cell recognition and signaling and for cell movement. Moesin contains 1 FERM domain and is expressed in all tissues and cultured cells studied. Moesin has been shown to interact with CD43, Neutrophil cytosolic factor 1, VCAM-1, Neutrophil cytosolic factor 4, ICAM3 and EZR. |
| Reference |
