Human N-Cadherin / CD325 / CDH2D Protein (His Tag)
CD325,CDHN,CDw325,NCAD
- 100ug (NPP4099) Please inquiry
| Catalog Number | P11039-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | CD325,CDHN,CDw325,NCAD |
| Molecular Weight | The pro form of human CDH2 consists of 710 amino acids and predictes a molecular mass of 78.5 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of CDH2 is 90 & 75 kDa corresponding to the pro fom and mature form respectinely due to glycosylation. |
| predicted N | Ser 26 |
| SDS-PAGE | 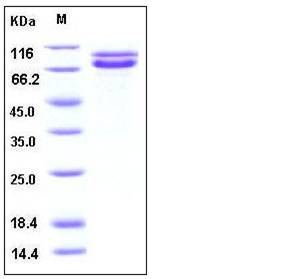 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the extracellular domain of human CDH2 (NP_001783.2) precursor (Met 1-Ala 724) was fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | Measured by the ability of the immobilized protein to support the adhesion of MCF-7 human breast adenocarcinoma cells. When 5 x 10E4 cells/well are added to Recombinant Human Cadherin-2 coated plates (5 μg/mL with 100 μL/well), approximately >50% will adhere after 1 hour at 37℃. |
| Research Area | Signaling |Signal Transduction |Cytoskeleton / ECM |Cell Adhesion |Cadherins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Cadherins are calcium dependent cell adhesion proteins, and they preferentially interact with themselves in a homophilic manner in connecting cells. Cadherin 2 (CDH2), also known as N-Cadherin (neuronal) (NCAD), is a single-pass tranmembrane protein and a cadherin containing 5 cadherin domains. N-Cadherin displays a ubiquitous expression pattern but with different expression levels between endocrine cell types. CDH2 (NCAD) has been shown to play an essential role in normal neuronal development, which is implicated in an array of processes including neuronal differentiation and migration, and axon growth and fasciculation. In addition, N-Cadherin expression was upregulated in human HSC during activation in culture, and function or expression blocking of N-Cadherin promoted apoptosis. During apoptosis, N-Cadherin was cleaved into 20-100 kDa fragments. It may provide a novel target for therapies that are directed toward intimal proliferative disorders, including restenosis and vascular bypass graft failure. N-Cadherin is associated with tumor aggressiveness and metastatic potential and may contribute to tumor progression. |
| Reference |
