Human NEIL1 Protein (His Tag)
FPG1,hFPG1,NEI1
- 100ug (NPP4105) Please inquiry
| Catalog Number | P12695-H08E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | FPG1,hFPG1,NEI1 |
| Molecular Weight | The recombinant human NEIL1 comprises 400 amino acids and migrates as an approximately 45 kDa band as predicted in SDS-PAGE under reducing conditions. |
| predicted N | Met 1 |
| SDS-PAGE | 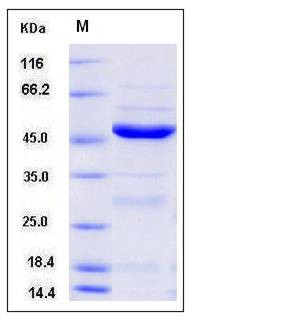 |
| Purity | > 84 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human NEIL1 (AAH10876.1) (Met 1-Ser 390) was fused with a polyhistidine tag at the C-terminus and an initial Met at the N-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile 50mM Tris, 150mM NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | NEIL1 is a member of DNA glycosylases. DNA glycosylases are a family homologous to the bacterial Fpg/Nei family. They play a role in base excision repair which is the mechanism by which damaged bases in DNA are removed and replaced. The first step of this process is catalyzed by DNA glycosylases. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site. NEIL1 functions in base excision repair of DNA damaged by oxidation or by mutagenic agents. It acts as DNA glycosylase that recognizes and removes damaged bases. NEIL1 prefers to oxidized pyrimidines, such as thymine glycol, formamidopyrimidine (Fapy) and 5-hydroxyuracil. Has marginal activity towards 8-oxoguanine. It has AP (apurinic/apyrimidinic) lyase activity and introduces nicks in the DNA strand and cleaves the DNA backbone by beta-delta elimination to generate a single-strand break at the site of the removed base with both 3'- and 5'-phosphates. |
| Reference |
