Human OPALIN Protein (Fc Tag)
HTMP10,OPALIN,TMEM10,TMP10
- 100ug (NPP4129) Please inquiry
| Catalog Number | P13999-H04H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | HTMP10,OPALIN,TMEM10,TMP10 |
| Molecular Weight | The recombinant human OPALIN/mFc comprises 327 amino acids and has a predicted molecular mass of 37.4 kDa. The apparent molecular mass of the monomer is approximately 44 and 33 kDa in SDS-PAGE under reducing conditions due to glycosylation. |
| predicted N | Asp |
| SDS-PAGE | 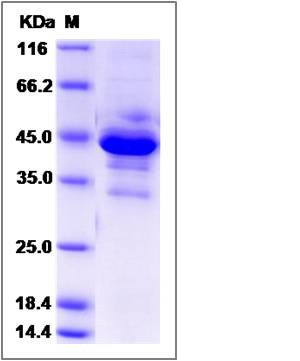 |
| Purity | (80.7+11.2) % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human OPALIN (Q96PE5-2) (Thr51-Glu141) was expressed with the Fc region of mouse IgG1 at the N-terminus. |
| Bio-activity | |
| Research Area | Neuroscience |Neurotransmission |Receptors / Channels |G Protein-Coupled Receptor (GPCR) |Opioid Receptors |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Opalin, or oligodendrocytic myelin paranodal and inner loop protein, is a transmembrane protein detected specifically in mammalian oligodendrocytes, and may play significant role in oligodendrocyte differentiation and myelination.Opalin has binding sites for Myt1 and cAMP-response element binding protein (CREB). Over-expression of Myt1, treatment of the cell with leukemia inhibitory factor (LIF), and cAMP analog (CREB activator) enhanced the expression of endogenous Opalin in Oli-neu cells and activated the oligodendrocyte enhancer. Thus LIF, cAMP signaling cascades and Myt1 may play significant roles in the differentiation of oligodendrocytes through their action on the Opalin oligodendrocyte enhancer. Enzymatic deglycosylation showed that myelin Opalin contained N- and O-glycans, and that the O-glycans, at least, had negatively charged sialic acids. Site-directed mutations at the glycan sites impaired the cell surface localization of Opalin. In addition to the somata and processes of oligodendrocytes, Opalin immunoreactivity was observed in myelinated axons in a spiral fashion, and was concentrated in the paranodal loop region. Immunogold electron microscopy demonstrated that Opalin was localized at particular sites in the paranodal loop membrane. These results suggest a role for highly sialylglycosylated Opalin in an intermembranous function of the myelin paranodal loops in the central nervous system. |
| Reference |
