Human ORP150 / HYOU1 / HSP12A Protein (His Tag)
GRP-170,Grp170,HSP12A,ORP-150,ORP150
- 100ug (NPP4130) Please inquiry
| Catalog Number | P11342-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | GRP-170,Grp170,HSP12A,ORP-150,ORP150 |
| Molecular Weight | The recombinant human HSP12A consists of 311 amino acids and predictes a molecular mass of 35.2 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rh HSP12A is approximately 55-65 kDa due to glycosylation. |
| predicted N | Met 695 |
| SDS-PAGE | 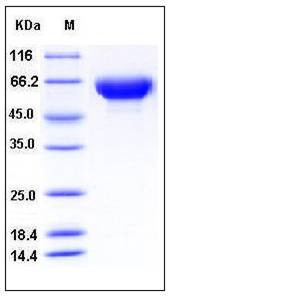 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the C-terminal segment of human HSP12A (NP_001124463.1) (Met 695-Leu 994) was expressed, fused with a polyhistidine tag at the C-terminus and a signal peptide at the N-terminus. |
| Bio-activity | |
| Research Area | Signaling |Signal Transduction |Metabolism |Pathways and Processes |Metabolism processes |Hypoxia | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Hypoxia up-regulated protein 1, also known as 150 kDa oxygen-regulated protein, 170 kDa glucose-regulated protein, ORP-150, GRP-170 and HYOU1, is a member of the heat shock protein 70 family. Seven members from four different heat shock protein (HSP) families were identified including HYOU1 (ORP150), HSPC1 (HSP86), HSPA5 (Bip), HSPD1 (HSP60), and several isoforms of the two testis-specific HSP70 chaperones HSPA2 and HSPA1L. HYOU1 is highly expressed in tissues that contain well-developed endoplasmic reticulum and synthesize large amounts of secretory proteins. It is highly expressed in liver and pancreas. HYOU1 is also expressed in macrophages within aortic atherosclerotic plaques, and in breast cancers. HYOU1 has a pivotal role in cytoprotective cellular mechanisms triggered by oxygen deprivation. It may play a role as a molecular chaperone and participate in protein folding. Suppression of HYOU1 is associated with accelerated apoptosis. It is suggested to have an important cytoprotective role in hypoxia-induced cellular perturbation. This protein has been shown to be up-regulated in tumors, especially in breast tumors, and thus it is associated with tumor invasiveness. |
| Reference |
