Human OTUB1 / OTB1 Protein (His Tag)
HSPC263,OTB1,OTU1
- 100ug (NPP4136) Please inquiry
| Catalog Number | P12927-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | HSPC263,OTB1,OTU1 |
| Molecular Weight | The recombinant human OTUB1 consisting of 282 amino acids and has a calculated molecular mass of 32.8 kDa. The apparent molecular mass of the protein is approximately 37 kDa in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 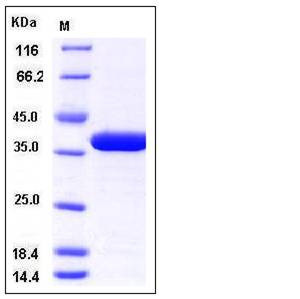 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human OTUB1 (Q96FW1-1) (Met 1-Lys 271) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Signaling Pathway |Representative pathway |Wnt Signaling pathway |Wnt Intracellular Signaling | |
| Formulation | Lyophilized from sterile PBS, 20% glycerol, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Ubiquitin thioesterase OTUB1, also known as Deubiquitinating enzyme OTUB1, OTU domain-containing ubiquitin aldehyde-binding protein 1, Otubain-1, Ubiquitin-specific-processing protease OTUB1, OTUB1 and OTB1, is a cytoplasm protein which belongs to the peptidase C65 family. OTUB1 is a hydrolase that can remove conjugated ubiquitin from proteins and plays an important regulatory role at the level of protein turnover by preventing degradation. OTUB1 is a regulator of T-cell anergy, a phenomenon that occurs when T-cells are rendered unresponsive to antigen rechallenge and no longer respond to their cognate antigen. OTUB1 acts via its interaction with RNF128 / GRAIL, a crucial inductor of CD4 T-cell anergy. Isoform 1 of OTUB1 destabilizes RNF128, leading to prevent anergy. In contrast, isoform 2 of OTUB1 stabilizes RNF128 and promotes anergy. OTUB1 regulates RNF128-mediated ubiquitination, but does not deubiquitinate polyubiquitinated RNF128. Deubiquitinates estrogen receptor alpha (ESR1). OTUB1 mediates deubiquitination of 'Lys-48'-linked polyubiquitin chains, but not 'Lys-63'-linked polyubiquitin chains. OTUB1 is also capable of removing NEDD8 from NEDD8 conjugates, but with a much lower preference compared to 'Lys-48'-linked ubiquitin. |
| Reference |
