Human Osteonectin / SPARC Protein (His Tag)
BM-40,ON,SPARC
- 100ug (NPP4132) Please inquiry
| Catalog Number | P10929-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | BM-40,ON,SPARC |
| Molecular Weight | The secreted recombinant human SPARC consists of 297 amino acids with the predicted molecular mass of 34 kDa. As a result of glycosylation, rhSPARC migrates as an approximately 45 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ala 18 |
| SDS-PAGE | 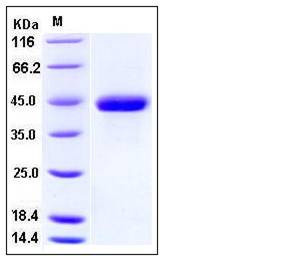 |
| Purity | > 98 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SPARC (NP_003109.1) precursor (Met 1-Ile 303) with a carboxy-terminal polyhistidine tag was expressed. |
| Bio-activity | |
| Research Area | Developmental Biology |Organogenesis |Skeletal development |Osteoblast and Osteoclast Markers |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Secreted protein acidic and rich in cysteine (SPARC), also known as Osteonectin (ON), is a member of the SPARC family. SPARC consists of three domains: a EF-hand domain, a follistatin-like domain and a Kazal-like domain, and each of which has independent activity and unique properties. The activity of SPARC is context- and cell-type-dependent, which is highlighted by the fact that SPARC has shown seemingly contradictory effects on tumor progression in both clinical correlative studies and in animal models. The location of SPARC in the nuclear matrix of certain proliferating cells, but only in the cytosol of postmitotic neurons, indicates potential functions of SPARC as a nuclear protein, which might be involved in the regulation of cell cycle progression and mitosis. It functions not only to modulate cell-cell and cell-matrix interactions, but its de-adhesive and growth inhibitory properties in non-transformed cells have led to studies to assess its role in cancer. Its divergent actions reflect the complexity of this protein, because in certain types of cancers, such as melanomas and gliomas, SPARC is associated with a highly aggressive tumor phenotype, while in others, mainly ovarian, neuroblastomas and colorectal cancers, SPARC may function as a tumor suppressor. Recent studies have also demonstrated a role for SPARC in sensitizing therapy-resistant cancers. Notably, SPARC is linked to human obesity. |
| Reference |
