Human P4HB / ERBA2L Protein (His Tag)
DSI,ERBA2L,GIT,P4Hbeta,PDI,PDIA1,PHDB,PO4DB,PO4HB,PROHB
- 100ug (NPP4138) Please inquiry
| Catalog Number | P10827-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | DSI,ERBA2L,GIT,P4Hbeta,PDI,PDIA1,PHDB,PO4DB,PO4HB,PROHB |
| Molecular Weight | The recombinant human PH4B consists of 499 amino acids and has a predicted molecular mass of 56.4 kDa. In SDS-PAGE under reducing conditions, it migrates with an apparent molecular mass of 60 kDa due to glycosylation. |
| predicted N | Asp 18 |
| SDS-PAGE | 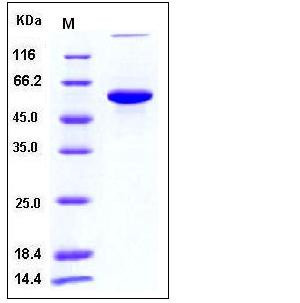 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PH4B (NP_000909.2) corresponding to amino acid (Met 1-Lys 505) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to promote aggregation of insulin in the presence of DTT. The specific activity is > 7.5 A650/min/mg |
| Research Area | Signaling |Signal Transduction |Cytoskeleton / ECM |Extracellular Matrix |ECM Proteins |Collagen | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Mouse protein disulfide-isomerase, also known as Cellular thyroid hormone-binding protein, Prolyl 4-hydroxylase subunit beta, p55 and P4HB, is a peripheral membrane protein which belongs to the protein disulfide isomerase family. P4HB is highly abundant. In some cell types, it seems to be also secreted or associated with the plasma membrane, where it undergoes constant shedding and replacement from intracellular sources. P4HB localizes near CD4-enriched regions on lymphoid cell surfaces. It is identified by mass spectrometry in melanosome fractions from stage I to stage IV. P4HB reduces and may activate fusogenic properties of HIV-1 gp120 surface protein, thereby enabling HIV-1 entry into the cell. P4HB catalyzes the formation, breakage and rearrangement of disulfide bonds. At the cell surface, it seems to act as a reductase that cleaves disulfide bonds of proteins attached to the cell. P4HB may therefore cause structural modifications of exofacial proteins. Inside the cell, it seems to form/rearrange disulfide bonds of nascent proteins. At high concentrations, P4HB functions as a chaperone that inhibits aggregation of misfolded proteins. At low concentrations, it facilitates aggregation (anti-chaperone activity). P4HB may be involved with other chaperones in the structural modification of the TG precursor in hormone biogenesis. It also acts a structural subunit of various enzymes such as prolyl 4-hydroxylase and microsomal triacylglycerol transfer protein MTTP. |
| Reference |
