Human PAPPA2 / Pappalysin 2 Protein (His Tag)
PAPP-A2,PAPP-E,PAPPE,PLAC3
- 100ug (NPP2382) Please inquiry
| Catalog Number | P10528-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | PAPP-A2,PAPP-E,PAPPE,PLAC3 |
| Molecular Weight | The secreted recombinant human PAPPA2 consists of 1174 amino acids with the predicted molecular mass of 131 kDa. As a result of glycosylation, rhPAPPA2 migrates as an approximately 170-180 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Ser 234 |
| SDS-PAGE | 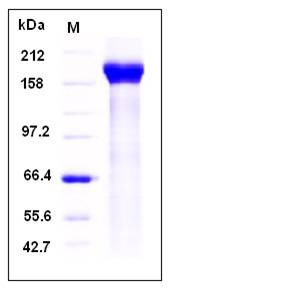 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PAPPA2 mature form (NP_064714.2) corresponding to amino acid (Ser 234-Cys 1396) was expressed, with a carboxy-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Pappalysin-2/PAPP-A2 is the second member of the pappalysin family of metzincin superfamily, of which PAPP-A is the first member. There is no homology between the prepro-peptides of PAPP-A and PAPP-A2, but 46% of the residues of mature PAPP-A are also present in mature PAPP-A2. PAPP-A specifically cleaves insulin-like growth factor-binding protein(IGFBP)-4, one of six known modulators of IGF-I and –II, whereas PAPP-A2 specifically cleaved IGFBP-5 at one site, between Ser-143 and Lys-144. In contrast to the cleavage of IGFBP-4 by PAPP-A that strictly requires the presence of IGF, the cleavage of IGFBP-5 by PAPP-A2 was IGF-independent. Recent data firmly establish PAPP-A and IGFBP-4 as an important functional pair in several systems. Because of its close relationship with PAPP-A, both structurally and functionally, PAPP-A2 is a likely candidate for IGFBP-5 proteinase in many tissues and conditioned media where IGFBP-5 proteolysis has been reported. |
| Reference |
