Human PKM2 / OIP3 Protein (His Tag)
CTHBP,HEL-S-30,OIP3,PK3,PKM2,TCB,THBP1
- 100ug (NPP4172) Please inquiry
| Catalog Number | P11430-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | CTHBP,HEL-S-30,OIP3,PK3,PKM2,TCB,THBP1 |
| Molecular Weight | The recombinant human PKM2 consisting of 541 amino acids and migrates as an approximately 59 kDa band in SDS-PAGE under reducing conditions as predicted. |
| predicted N | Met |
| SDS-PAGE | 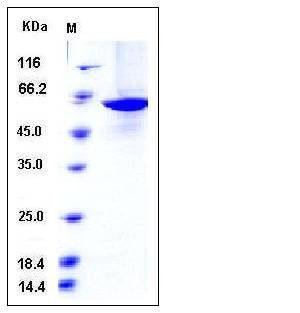 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PKM2 isoform M2 (P14618-1) (Ser 2-Pro 531) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | Kinase activity untested |
| Research Area | Cancer |Signal transduction |Metabolism |Types of disease |Metabolism in Cancer |
| Formulation | Supplied as sterile PBS, pH 7.0, 10% glycerol 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Pyruvate kinase isozymes M2 also known as pyruvate kinase muscle isozyme 2 (PKM2), pyruvate kinase type K, cytosolic thyroid hormone-binding protein (CTHBP), thyroid hormone-binding protein 1 (THBP1), or opa-interacting protein 3 (OIP3), is an isoenzyme of the glycolytic enzyme pyruvate kinase. Pyruvate kinase isozymes M2 / PKM2 is a protein involved in glycolysis. The encoded protein is a pyruvate kinase that catalyzes the transfer of a phosphoryl group from phosphoenolpyruvate to ADP, generating ATP and pyruvate. PKM2 has been shown to interact with thyroid hormone and may mediate cellular metabolic effects induced by thyroid hormones. PKM2 has been found to bind Opa protein, a bacterial outer membrane protein involved in gonococcal adherence to and invasion of human cells, suggesting a role of this protein in bacterial pathogenesis. Several alternatively spliced transcript variants encoding a few distinct isoforms have been reported. PKM2 functions as a glycolytic enzyme that catalyzes the transfer of a phosphoryl group from phosphoenolpyruvate (PEP) to ADP, generating ATP. PKM2 may stimulates POU5F1-mediated transcriptional activation. This protein Plays a general role in caspase independent cell death of tumor cells. The ratio betwween the highly active tetrameric form and nearly inactive dimeric form determines whether glucose carbons are channeled to biosynthetic processes or used for glycolytic ATP production. The transition between the 2 forms of PKM2 contributes to the control of glycolysis and is important for tumor cell proliferation and survival. |
| Reference |
