Human PMP2 / FABP8 Protein (His Tag)
FABP8,M-FABP,MP2,P2
- 100ug (NPP2416) Please inquiry
| Catalog Number | P11426-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | FABP8,M-FABP,MP2,P2 |
| Molecular Weight | The recombinant human PMP2 consisting of 138 amino acids and migrates as an approximately16 kDa band in SDS-PAGE under reducing conditions as predicted. |
| predicted N | Met 1 |
| SDS-PAGE | 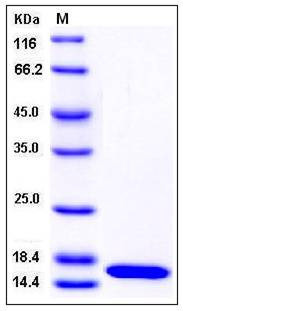 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PMP2 (NP_002668.1) (Ser 2-Val 132) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |DNA / RNA |Translation |Regulation |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Myelin P2 protein, also known as PMP2, is a cytosolic protein found primarily in peripheral nerves. It Belongs to the calycin superfamily. Fatty-acid binding protein (FABP) family. PMP2 is a small, basic, and cytoplasmic lipid binding protein of peripheral myelin. It is similar in amino acid sequence and tertiary structure to fatty acid binding proteins found in the liver, adipocytes, and intestine, its expression is limited to the nervous system. PMP2 is detected only in myelin-producing cells of the central and peripheral nervous systems, the oligodendrocytes and Schwann cells, respectively. PMP2 may play a role in lipid transport protein in Schwann cells. It forms a beta-barrel structure that accommodates hydrophobic ligands in its interior. |
| Reference |
