Human PNLIP Protein (His Tag)
PL,PNLIPD,PTL
- 100ug (NPP2417) Please inquiry
| Catalog Number | P13564-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | PL,PNLIPD,PTL |
| Molecular Weight | The recombinant human PNLIP consists of 460 amino acids and predicts a molecular mass of 51 KDa. It migrates as an approximately 51 KDa band in SDS-PAGE under reducing conditions. |
| predicted N | Lys 17 |
| SDS-PAGE | 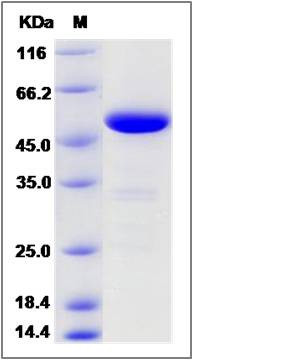 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PNLIP (P16233) (Met1-Cys465) was expressed with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Cancer |Signal transduction |Metabolism |Pathways and Processes |Metabolic signaling pathways |Lipid and lipoprotein metabolism | |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | PNLIP is an enzyme which belongs to the lipase family. Secreted from the pancreas, PNLIP is the primary lipase that hydrolyzes dietary fat molecules in the human digestive system, converting triglyceride substrates found in ingested oils to monoglycerides and free fatty acids. Bile salts secreted from the liver and stored in gallbladder are released into the duodenum where they coat and emulsify large fat droplets into smaller droplets, thus increasing the overall surface area of the fat, which allows the lipase to break apart the fat more effectively. The resulting monomers (2 free fatty acids and one 2-monoacylglycerol) are then moved by way of peristalsis along the small intestine to be absorbed into the lymphatic system by a specialized vessel called a lacteal. |
| Reference |
