Human PPIase / FKBP7 Protein (Fc Tag)
FKBP23,PPIase
- 100ug (NPP4183) Please inquiry
| Catalog Number | P13250-H02H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | FKBP23,PPIase |
| Molecular Weight | The recombinant human FKBP7/Fc is a disulfide-linked homodimer. The reduced monomer comprises 436 amino acids and has a predicted molecular mass of 49.4 kDa. The apparent molecular mass of the protein is approximately 55kDa in SDS-PAGE under reducing conditions. |
| predicted N | Gln 24 |
| SDS-PAGE | 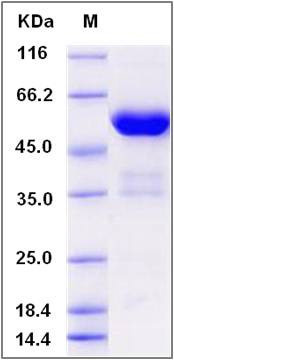 |
| Purity | > 90 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human FKBP7 (Q9Y680-2) (Met1-Gln218) was expressed, fused with the Fc region of human IgG1 at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Protein Trafficking |ER Proteins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | PPIase is a member of the immunophilin protein family. It also belongs to the cyclophilin-type PPIase family, PPIL3 subfamily. PPIase contains 1 PPIase cyclophilin-type domain. Members of the immunophilin protein family play a role in immunoregulation and basic cellular processes involving protein folding and trafficking. PPIases accelerate the folding of proteins. It catalyzes the cis-trans isomerization of proline imidic peptide bonds in oligopeptides. It has a very high substrate specificity for the four-residue peptide Ala-Ala-Pro-Phe only when the proline peptide bond is in the trans state. It interacts with several intracellular signal transduction proteins including type I TGF-beta receptor. It also interacts with multiple intracellular calcium release channels, and coordinates multi-protein complex formation of the tetrameric skeletal muscle ryanodine receptor. In mouse, deletion of this homologous gene causes congenital heart disorder known as noncompaction of left ventricular myocardium. |
| Reference |
