Human PRMT3 Protein (GST Tag)
HRMT1L3
- 100ug (NPP4187) Please inquiry
| Catalog Number | P11211-H09E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | HRMT1L3 |
| Molecular Weight | The recombinant human PRMT3/GST chimera consists of 754 amino acids and predicts a molecular mass of 85.7 kDa. It migrates as an approxiamtely 65 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 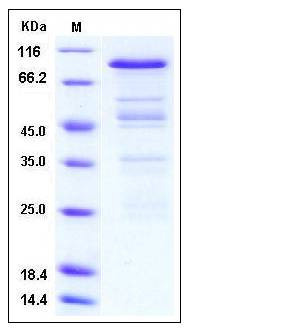 |
| Purity | > 65 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PRMT3 (NP_005779.1) (Cys 2-Gln 531) was fused with the GST tag at the N-terminus. |
| Bio-activity | |
| Research Area | |
| Formulation | Lyophilized from sterile 20mM Tris, 150mM NaCl, 0.5mM GSH, pH 7.0 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Protein arginine methyltransferase 3, also known as PRMT3, is one of four type I protein arginine methyltransferases (PRMT) that in humans is encoded by the PRMT3 gene. Methylation of arginine residues is a widespread post-translational modification of proteins catalyzed by a small family of PRMTs. The modification appears to regulate protein functions and interactions that affect gene regulation, signalling and subcellular localization of proteins and nucleic acids. In human cells, the PRMT family consists of eight canonical members. PRMTs have been classified into two groups based on the end product. Certain PRMTs display different subcellular localization in different cell types, implicating cell- and tissue-specific mechanisms for regulating PRMT functions. PRMT3 is unique in that its N-terminus harbours a C2H2 zinc-finger domain that is proposed to confer substrate specificity. In addition, PRMT3 is the only type I enzyme that is restricted to the cytoplasm. A large proportion of this cystosolic PRMT3 is found associated with ribosomes. It is tethered to the ribosomes through its interaction with rpS2, which is also its substrate. |
| Reference |
