Human PRMT5 / SKB1 Protein (His & FLAG Tag)
HRMT1L5,IBP72,JBP1,SKB1,SKB1Hs
- 100ug (NPP4188) Please inquiry
| Catalog Number | P11074-H18H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | HRMT1L5,IBP72,JBP1,SKB1,SKB1Hs |
| Molecular Weight | The recombinant human PRMT5 consists of 655 amino acids and predictes a molecular mass of 75 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhPRMT5 is approximately 65 kDa. |
| predicted N | Met |
| SDS-PAGE | 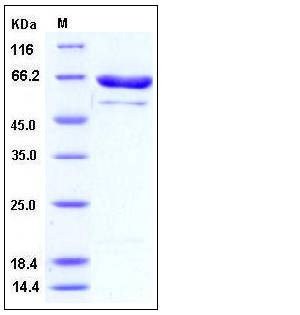 |
| Purity | > 85 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PRMT5 (NP_006100.2) (Ala 2-Leu 637) was expressed, fused with a polyhistidine tag at the C-terminus and a Flag tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Histone Modifying Enzymes |Methylation and Demethylation |Histone methylation |
| Formulation | Lyophilized from sterile 50mM Tris, 100mM NaCl, pH 8.0, 0.5mM EDTA, 0.5PMSF, 0.5mM, TCEP, 25% glycerol 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Methylation of arginine residues is a widespread post-translational modification of proteins catalyzed by a small family of PRMTs. The modification appears to regulate protein functions and interactions that affect gene regulation, signalling and subcellular localization of proteins and nucleic acids. Protein arginine methyltransferase 5 (PRMT5) is a member of the protein arginine N-methyltransferases (PRMT)family, and exists as at least homodimers and homotetramers, or homooligomers mediated by disulfide bonds and non-covalent association ubiquitously. PRMT5 specifically mediates the symmetrical dimethylation of arginine residues in the small nuclear ribonucleoproteins Sm D1 (SNRPD1) and Sm D3 (SNRPD3), and thus plays a role in the assembly and biogenesis of snRNP core particles. PRMT5 methylates histone H2A and H4 'Arg-3' during germ cell development, as well as histone H3 'Arg-8', which may repress transcription. PRMT5 also methylates SUPT5H and regulates its transcriptional elongation properties. Additionally, it is also suggested that PRMT5 negatively regulates cyclin E1 promoter activity and cellular proliferation. |
| Reference |
