Human PRMT6 / HRMT1L6 Protein (His & FLAG Tag)
HRMT1L6
- 100ug (NPP4189) Please inquiry
| Catalog Number | P11313-H18H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | HRMT1L6 |
| Molecular Weight | The recombinant human PRMT6 consists of 394 amino acids and predictes a molecular mass of 44.4 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rh PRMT6 is approximately 43-46 kDa. |
| predicted N | Met |
| SDS-PAGE | 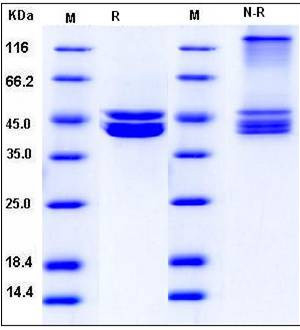 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human PRMT6 (NP_060607.2) (Met 1-Asp 375) was expressed, fused with a polyhistidine tag at the C-terminus and the flag tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |DNA / RNA |RNA Processing |Other in RNA processing |
| Formulation | Lyophilized from sterile PBS, pH 7.5 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Protein arginine N-methyltransferase 6, also known as Histone-arginine N-methyltransferase PRMT6, PRMT6, and HRMT1L6, is a member of the protein arginine N-methyltransferase family and PRMT6 subfamily. PRMT6 is highly expressed in kidney and testes. PRMT6 is known to catalyze the generation of asymmetric dimethylarginine in polypeptides. It has been implicated in human immunodeficiency virus pathogenesis, DNA repair, and transcriptional regulation. PRMT6 is known to methylate histone H3 Arg-2 (H3R2), and this negatively regulates the lysine methylation of H3K4 resulting in gene repression. PRMT6 plays a key role in coupling process by functioning as a transcriptional coactivator that can regulate alternative splicing. PRMT6 coactivates the progesterone, glucocorticoid and oestrogen receptors in luciferase reporter assays in a hormone-dependent manner. Small interfering RNA (siRNA) oligonucleotide duplex knockdown of PRMT6 disrupts oestrogen-stimulated transcription of endogenous GREB1 and progesterone receptor in MCF-7 breast cancer cells. Neutralizing the activity of PRMT6 could inhibit tumor progression and may be of cancer therapeutic significance. |
| Reference |
