Human RUVBL1 / RVB1 Protein (His Tag)
ECP54,INO80H,NMP238,PONTIN,Pontin52,RVB1,TIH1,TIP49,TIP49A
- 100ug (NPP2472) Please inquiry
| Catalog Number | P14074-H07B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | ECP54,INO80H,NMP238,PONTIN,Pontin52,RVB1,TIH1,TIP49,TIP49A |
| Molecular Weight | The recombinant human RUVBL1 consists of 474 amino acids and has a calculated molecular mass of 52.4 kDa. The recombinant protein migrates as an approximately 57 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | His |
| SDS-PAGE | 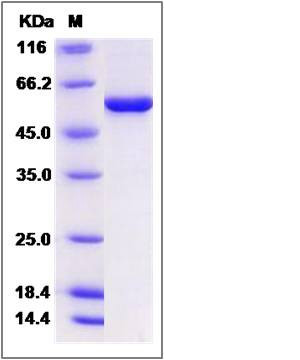 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human RUVBL1 (Q9Y265-1) (Met1-Lys456) was fused with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Transcription |Other factors |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM Nacl, pH 7.4, 10% glycerol 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | RUVBL1, also known as RVB1, is a component of the NuA4 histone acetyltransferase complex and belongs to the RuvB family. RUVBL1 is ubiquitously expressed with high expression in heart, skeletal muscle and testis. It possesses single-stranded DNA-stimulated ATPase and ATP-dependent DNA helicase (3' to 5') activity. RUVBL1 is involved in transcriptional activation of select genes principally by acetylation of nucleosomal histones H4 and H2A. RUVBL1 plays an essential role in oncogenic transformation by MYC and also modulates transcriptional activation by the LEF1/TCF1-CTNNB1 complex. It also is essential for cell proliferation. RUVBL1 may be able to bind plasminogen at cell surface and enhance plasminogen activation. |
| Reference |
