Human SETD7 / SET7/9 Protein (His Tag)
KMT7,SET7,SET7/9,SET9
- 100ug (NPP2495) Please inquiry
| Catalog Number | P11209-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | KMT7,SET7,SET7/9,SET9 |
| Molecular Weight | The recombinant human SETD7 comprises 372 amino acids and has a predicted molecular mass of 41.5 kDa. It migrates as an approximately 48 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 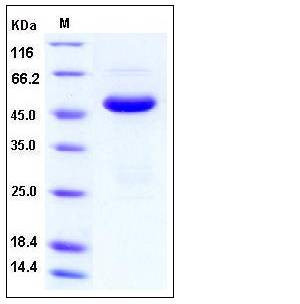 |
| Purity | > 94 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SETD7 (NP_085151.1) (Asp 2-Lys 366) was expressed, with a polyhistidine tag at the N-terminus. |
| Bio-activity | |
| Research Area | Epigenetics |Histone Modifying Enzymes |Methylation and Demethylation |Histone methylation |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Histone-lysine N-methyltransferase SETD7, also known as SET domain containing (lysine methyltransferase) 7, SET7/9, Histone H3-K4 methyltransferase SETD7, H3-K4-HMTase SETD7, and SETD7, is a member of the histone-lysine methyltransferase family and SET7 subfamily. SETD7 is widely expressed and expressed in pancreatic islets. SETD7 contains three MORN repeats and one SET domain. SETD7 plays a central role in the transcriptional activation of genes such as collagenase or insulin. As a protein lysine methyltransferase (PKMT), SETD7 also has methyltransferase activity toward non-histone proteins such as p53/TP53, TAF10, and possibly TAF7 by recognizing and binding in substrate proteins. The mono-methyltransferase activity of SETD7 is achieved by disrupting the formation at near-attack conformations for the dimethylation reaction. SETD7 is also a novel coactivator of NF-kappaB and plays a role in inflammation and diabetes. |
| Reference |
