Human SMOC1 Protein (His Tag)
OAS
- 100ug (NPP2506) Please inquiry
| Catalog Number | P12026-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | OAS |
| Molecular Weight | The recombinant human SMOC1 consists of 420 amino acids and has a predicted molecular mass of 47 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhSMOC1 is approximately 55-60 kDa due to glycosylation. |
| predicted N | His 27 |
| SDS-PAGE | 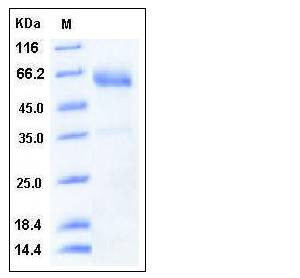 |
| Purity | > 92 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SMOC1 isoform 1 (NP_001030024.1) (Met 1-Val 435) was expressed, fused with a polyhistidine tag at the C-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Signaling Pathway |Calcium Signaling |Calcium Binding Proteins |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SPARC-related modular calcium-binding protein 1, also known as secreted modular calcium-binding protein 1 and SMOC1, is a member of the SPARC family. SMOC1 is widely expressed in many tissues with a strongest signal in ovary. It contains two EF-hand domains, one Kazal-like domain and two thyroglobulin type-1 domains. Extracellular matrix proteins have been implicated in the regulation of osteoblast differentiation of bone marrow derived mesenchymal stem cells (BMSCs) through paracrine or autocrine mechanisms. SMOC1 is a regulator of osteoblast differentiation of BMSCs. SMOC1 is highly expressed and secreted in BMSCs stimulated with osteogenic medium (OSM). SMOC1 and SMOC2 are matricellular proteins thought to influence growth factor signaling, migration, proliferation, and angiogenesis. SMOC1 and SMOC2 may mediate intercellular signaling and cell type-specific differentiation during gonad and reproductive tract development. |
| Reference |
