Human SMYD3 / ZMYND1 Protein (GST Tag)
bA74P14.1,KMT3E,ZMYND1,ZNFN3A1
- 100ug (NPP4280) Please inquiry
| Catalog Number | P11217-H09B |
|---|---|
| Organism Species | Human |
| Host | Baculovirus-Insect Cells |
| Synonyms | bA74P14.1,KMT3E,ZMYND1,ZNFN3A1 |
| Molecular Weight | The recombinant human SMYD3/GST chimera consists of 559 amino acids and predicts a molecular mass of 656 kDa. It migrates as an approxiamtely 58 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 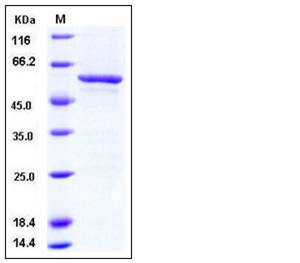 |
| Purity | > 88 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SMYD3 isoform 2 (NP_073580.1) (Lys 35-Ser 369) was fused with the GST tag at the N-terminus. |
| Bio-activity | |
| Research Area | Cancer |Oncoprotein & suppressor & biomarker |Oncoprotein |Transcription factor |
| Formulation | Lyophilized from sterile 20mM Tris, 150mM NaCl, 0.5mM DTT, 0.5mM GSH, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SET and MYND domain-containing protein 3, also known as Zinc finger MYND domain-containing protein 1, SMYD3, and ZMYND, is a member of the histone-lysine methyltransferase family. SMYD3 contains one MYND-type zinc finger and one SET domain. SMYD3 is a histone H3 lysine-4-specific methyltransferase. It is expressed in skeletal muscles and testis. It is overexpressed in a majority of colorectal carcinoma (CRC) and hepatocellular carcinoma (HCC). SMYD3 plays an important role in transcriptional regulation in human carcinogenesis. It activates the transcription of a set of downstream genes. Of these downstream genes, there are several oncogenes and genes associated with cell adhesion (including those of N-Myc, CrkL, Wnt10b, L-selectin, CD31 and galectin-4), which have been shown to have effects on cell viability, adhesion, migration and metastasis. Increased SMYD3 expression is essential for the proliferation of breast cancer cells. SMYD3 may be a promising new target of therapeutic intervention for the treatment of cancers or other pathological processes associated with cell adhesion and migration. |
| Reference |
