Human SOD1 / Superoxide Dismutase Protein (His Tag)
ALS,ALS1,HEL-S-44,homodimer,hSod1,IPOA,SOD
- 100ug (NPP4285) Please inquiry
| Catalog Number | P11727-H07E |
|---|---|
| Organism Species | Human |
| Host | E. coli |
| Synonyms | ALS,ALS1,HEL-S-44,homodimer,hSod1,IPOA,SOD |
| Molecular Weight | The recombinant human SOD1 consisting of 161 amino acids and has a calculated molecular mass of 16.8 kDa. It migrates as an approximately 20 kDa band in SDS-PAGE under reducing conditions. |
| predicted N | Met |
| SDS-PAGE | 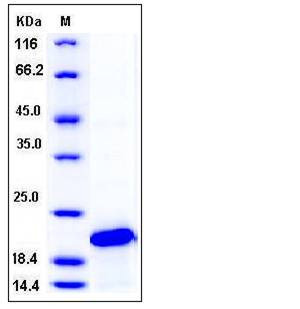 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SOD1 (NP_000445.1) (Ala 2-Gln 154) was expressed, with a polyhistide tag at the N-terminus. |
| Bio-activity | |
| Research Area | Immunology |Signal Transduction |Cellular Senescence and Pathways in Aging |Apoptosis |Oxidative Stress |Anti-oxidant Molecules | |
| Formulation | Lyophilized from sterile 20mM Tris, 500mM NaCl, pH 8.0 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SOD1 belongs to the Cu-Zn superoxide dismutase family. It binds copper and zinc ions and is one of two isozymes responsible for destroying free superoxide radicals in the body. The encoded isozyme is a soluble cytoplasmic protein, acting as a homodimer to convert naturally-occuring but harmful superoxide radicals to molecular oxygen and hydrogen peroxide. The other isozyme is a mitochondrial protein. Mutations in this gene have been implicated as causes of familial amyotrophic lateral sclerosis. Rare transcript variants have been reported for this gene. SOD1 destroys radicals which are normally produced within the cells and which are toxic to biological systems. Defects in SOD1 are the cause of amyotrophic lateral sclerosis type 1 (ALS1). ALS1 is a familial form of amyotrophic lateral sclerosis, a neurodegenerative disorder affecting upper and lower motor neurons and resulting in fatal paralysis. Sensory abnormalities are absent. Death usually occurs within 2 to 5 years. The etiology of amyotrophic lateral sclerosis is likely to be multifactorial, involving both genetic and environmental factors. The disease is inherited in 5-10% of cases leading to familial forms. |
| Reference |
