Human SerpinE1 / PAI-1 Protein (His Tag)
PAI,PAI-1,PAI1,PLANH1,SERPINE1
- 100ug (NPP4141) Please inquiry
| Catalog Number | P10296-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | PAI,PAI-1,PAI1,PLANH1,SERPINE1 |
| Molecular Weight | The mature recombinant human SerpinE1 consists of 390 amino acids and predicts a molecular mass of 44.2 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rhSerpinE1 is approximately 45 kDa. |
| predicted N | Val 24 |
| SDS-PAGE | 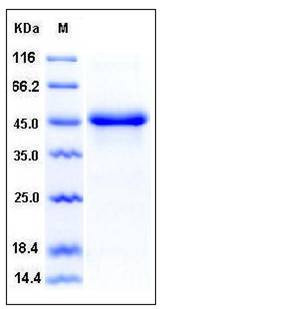 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SerpinE1 precursor (NP_000593.1) (Met 1-Pro 402) was expressed with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to inhibit uPA cleavage of a peptide substrate, N-carbobenzyloxy-Gly-Gly-Arg-7-amido-4-methylcoumarin (Z-GGR-AMC). The IC50 value is < 60 nM. |
| Research Area | Immunology |Signal Transduction |Cellular Senescence and Pathways in Aging |DNA Damage and Repair |
| Formulation | Lyophilized from sterile 50mM NaAc, 0.1M NaCl, pH 5.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Plasminogen activator inhibitor 1, also known as PAI-1, Endothelial plasminogen activator inhibitor, SerpinE1 and PLANH1, is a secreted glycoprotein which belongs to the serpin family. SerpinE1 is the primary physiological inhibitor of the two plasminogen activators urokinase (uPA) and tissue plasminogen activator (tPA). Its rapid interaction with TPA may function as a major control point in the regulation of fibrinolysis. Defects in SerpinE1 are the cause of plasminogen activator inhibitor-1 deficiency (PAI-1 deficiency) which is characterized by abnormal bleeding due to SerpinE1 defect in the plasma. High concentrations of SerpinE1 have been associated with thrombophilia which is an autosomal dominant disorder in which affected individuals are prone to develop serious spontaneous thrombosis. Studies of PAI-1 have contributed significantly to the elucidation of the protease inhibitory mechanism of serpins, which is based on a metastable native state becoming stabilised by insertion of the RCL into the central beta-sheet A and formation of covalent complexes with target proteases. Greater expression of PAI-1 has been associated with increased survival of cells and resistance to apoptosis. PAI-1 appears to influence apoptosis by decreasing cell adhesion (anoikis) as well as its effect on intracellular signaling. PAI-1, in its active state, also binds to the extracellular protein vitronectin. When in complex with its target proteases, it binds with high affinity to endocytosis receptors of the low density receptor family. The mechanisms of PAI-1 overexpression during obesity are complex, and it is conceivable that several inducers are involved at the same time at several sites of synthesis. PAI-1 is also implicated in adipose tissue development. It suggests that PAI-1 inhibitors serve in the control of atherothrombosis. |
| Reference |
