Human SerpinF2 / SERPINF2 Protein (His Tag)
A2AP,AAP,ALPHA-2-PI,API,PLI
- 100ug (NPP4260) Please inquiry
| Catalog Number | P10297-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | A2AP,AAP,ALPHA-2-PI,API,PLI |
| Molecular Weight | The recombinant human SerpinF2 consists of 475 amino acids after removal of the signal peptide and predicts a molecular mass of 53.2 kDa. By SDS-PAGE under reducing conditions, the apparent molecular mass of rhSerpinF2 is approximately 70 kDa due to glycosylation. |
| predicted N | Met 28 |
| SDS-PAGE | 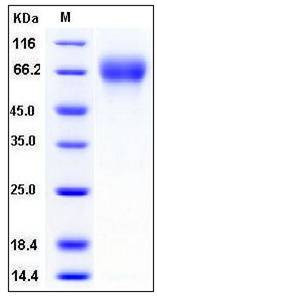 |
| Purity | > 97 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SerpinF2 (NP_000925.2) (Met 1-Lys 491) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | Measured by its ability to inhibit trypsin cleavage of a fluorogenic peptide substrate,Mca-RPKPVE-Nval-WRK(Dnp)-NH2 (Anaspec, Catalog#27114) . The IC50 value is < 0.5 nM . |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Complement System |Regulatory |
| Formulation | Lyophilized from sterile 25mM Tris, 150mM NaCl, pH 7.5 1. Normally 5 % - 8 % trehalose and mannitol are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | SerpinF2, also known as alpha-2 antiplasmin (alpha-2 AP), is a member of the Serpin superfamily. SerpinF2 is the principal physiological inhibitor of serine protease plasmin, and as well as, an efficient inhibitor of trypsin and chymotrypsin. This protease is produced mainly by liver and kidney, and also expressed in muscle, intestine, central nervous system, and placenta also express this protein at a moderate level. It is indicated that Serpin F2 is a key regulator of plasmin-mediated proteolysis in these tissues. Alpha-2 AP is an unusual serpin in that it contains extensive N- and C-terminal sequences flanking the serpin domain. The N-terminal sequence is crosslinked to fibrin by factor XIIIa, whereas the C-terminal region mediates the initial interaction with plasmin. SerpinF2 is one of the inhibitors of fibrinolysis, which acts as the primary inhibitor of plasmin(ogen). It is a specific plasmin inhibitor, and is important in modulating the effectiveness and persistence of fibrin with respect to its susceptibility to digestion and removal by plasmin. Alpha-2 AP plays the dominant role in inhibiting both plasma clot lysis and thrombus lysis, and accordingly, the congenital deficiency of Alpha-2 antiplasmin causes a rare bleeding disorder because of increased fibrinolysis. Thus, it may be a useful target for developing more effective treatment of thrombotic diseases. |
| Reference |
