Human SerpinI1 / Neuroserpin Protein (His Tag)
neuroserpin,PI12
- 100ug (NPP4113) Please inquiry
| Catalog Number | P11107-H08H |
|---|---|
| Organism Species | Human |
| Host | Human Cells |
| Synonyms | neuroserpin,PI12 |
| Molecular Weight | The secreted recombinant human SERPINI1 consists of 405 amino acids after removal of the signal peptide and has a calculated molecular mass of 46 kDa. In SDS-PAGE under reducing conditions, the apparent molecular mass of rh SERPINI1 is approximately 50-55 kDa due to glycosylation. |
| predicted N | Thr 17 |
| SDS-PAGE | 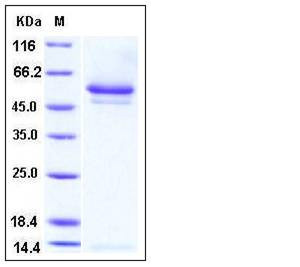 |
| Purity | > 95 % as determined by SDS-PAGE |
| Protein Construction | A DNA sequence encoding the human SERPINI1 (NP_005016.1) (Met 1-Leu 410) was expressed, with a C-terminal polyhistidine tag. |
| Bio-activity | |
| Research Area | Immunology |Inflammation / Inflammatory Mediator |Plasma Cascade Systems in Inflammation |Fibrinolysis System |
| Formulation | Lyophilized from sterile PBS, pH 7.4 1. Normally 5 % - 8 % trehalose, mannitol and 0.01% Tween80 are added as protectants before lyophilization. Specific concentrations are included in the hardcopy of COA. |
| Background | Neuroserpin, also known as Protease inhibitor 12 and SERPINI1, is a secreted protein which belongs to the serpin family. Neuroserpin is a serine protease inhibitor that inhibits plasminogen activators and plasmin but not thrombin. Serine protease inhibitors of the serpin superfamily are involved in many cellular processes. Neuroserpin was first identified as a protein secreted from the axons of dorsal root ganglion neurons. Neuroserpin is predominantly expressed in the brain, and is expressed in the late stages of neurogenesis during the process of synapse formation. Overexpression of neuroserpin in an anterior pituitary corticotroph cell line results in the extension of neurite-like processes, suggesting that neuroserpin may play a role in cell communication, cell adhesion, and/or cell migration. Neuroserpin may be involved in the formation or reorganization of synaptic connections, as well as synaptic plasticity in the adult nervous system. Neuroserpin may also protect neurons from cell damage by tissue-type plasminogen activator. Defects of neuroserpin are the cause of familial encephalopathy with neuroserpin inclusion bodies (FEN1B). |
| Reference |
